Human TNF RI/TNFRSF1A Antibody Summary
Accession # NP_001056
Applications
The ED50 for this effect is typically 1-6 μg/mL.
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
TNF RI/TNFRSF1A in Human Peripheral Blood Lymphocytes. TNF RI/TNFRSF1A was detected in immersion fixed human peripheral blood lymphocytes using 5 µg/mL Human TNF RI/TNFRSF1A Antigen Affinity-purified Polyclonal Antibody (Catalog # AF225) for 3 hours at room temperature. Cells were stained (red) and counterstained (green). View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
 View Larger
View Larger
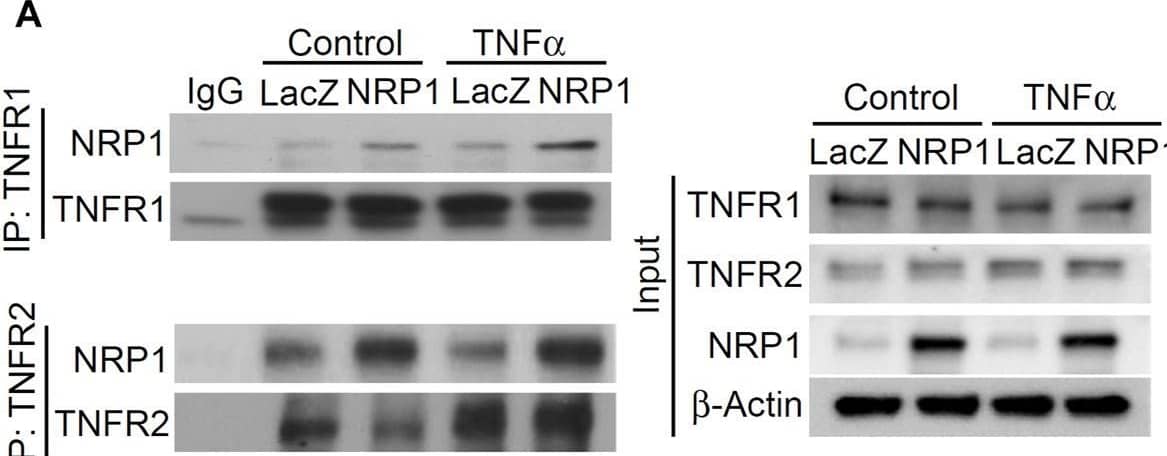
Detection of Human TNF RI/TNFRSF1A by Western Blot NRP1 and TNFRs are in the same protein complexes. (A) HUVECs were infected with retrovirus expressing NRP1 or LacZ as a control, and then stimulated with TNF alpha (5 ng/mL, 15 min). Cell lysates were immunoprecipated with anti-TNFR1 or TNFR2 antibodies and immunoprecipates were analyzed for the presence of the TNFRs and NRP1 by Western blotting (left panels). Cell lysates were immunoblotted as load control (right panel). (B) HUVECs were infected lentivirus expressing control-shRNA and NRP1-shRNA, respectively, selected with puromycin for 48 h, stimulated with TNF alpha and then subjected to Western blotting. (C, D) HUVECs (5*104/well) were plated into a 24-well plate. Half of the wells were pre-incubated with an excess of “non-GpL” TNF alpha (2 μg/mL) to determine the non-specific binding of Gaussia. princeps luciferase TNF alpha (GpL-TNF alpha ). Then all the wells were incubated with GpL-TNF alpha at different concentrations for 30 min at 37°C. The non-specific binding values of the “non-GpL” TNF alpha groups were subtracted from the corresponding total binding values. The data were analyzed by a non-linear regression to a single site using the GraphPad Prism 5 software. The experiments were performed in duplicates and independently repeated for 3 times. (E) HUVECs were stimulated with TNF alpha (5 ng/mL) for 15 min and then subjected to immunofluorescent staining with the indicated antibodies. Nuclei were counterstained with DAPI. Co-localization of NRP1 and TNFR1 in both plasmal membrane (arrow) and cytoplasm were observed. Scale bar, 10 μm. Image collected and cropped by CiteAb from the following open publication (https://www.frontiersin.org/articles/10.3389/fcell.2024.1210944/full), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
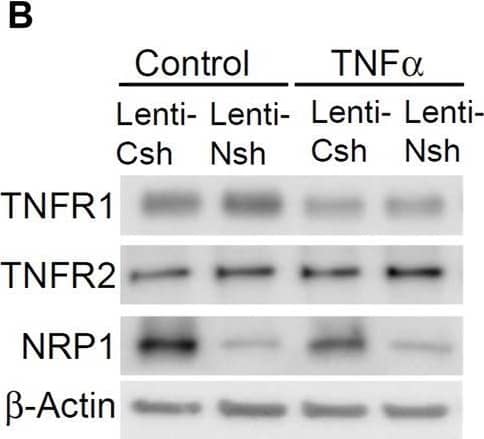
Detection of Human TNF RI/TNFRSF1A by Western Blot NRP1 and TNFRs are in the same protein complexes. (A) HUVECs were infected with retrovirus expressing NRP1 or LacZ as a control, and then stimulated with TNF alpha (5 ng/mL, 15 min). Cell lysates were immunoprecipated with anti-TNFR1 or TNFR2 antibodies and immunoprecipates were analyzed for the presence of the TNFRs and NRP1 by Western blotting (left panels). Cell lysates were immunoblotted as load control (right panel). (B) HUVECs were infected lentivirus expressing control-shRNA and NRP1-shRNA, respectively, selected with puromycin for 48 h, stimulated with TNF alpha and then subjected to Western blotting. (C, D) HUVECs (5*104/well) were plated into a 24-well plate. Half of the wells were pre-incubated with an excess of “non-GpL” TNF alpha (2 μg/mL) to determine the non-specific binding of Gaussia. princeps luciferase TNF alpha (GpL-TNF alpha ). Then all the wells were incubated with GpL-TNF alpha at different concentrations for 30 min at 37°C. The non-specific binding values of the “non-GpL” TNF alpha groups were subtracted from the corresponding total binding values. The data were analyzed by a non-linear regression to a single site using the GraphPad Prism 5 software. The experiments were performed in duplicates and independently repeated for 3 times. (E) HUVECs were stimulated with TNF alpha (5 ng/mL) for 15 min and then subjected to immunofluorescent staining with the indicated antibodies. Nuclei were counterstained with DAPI. Co-localization of NRP1 and TNFR1 in both plasmal membrane (arrow) and cytoplasm were observed. Scale bar, 10 μm. Image collected and cropped by CiteAb from the following open publication (https://www.frontiersin.org/articles/10.3389/fcell.2024.1210944/full), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
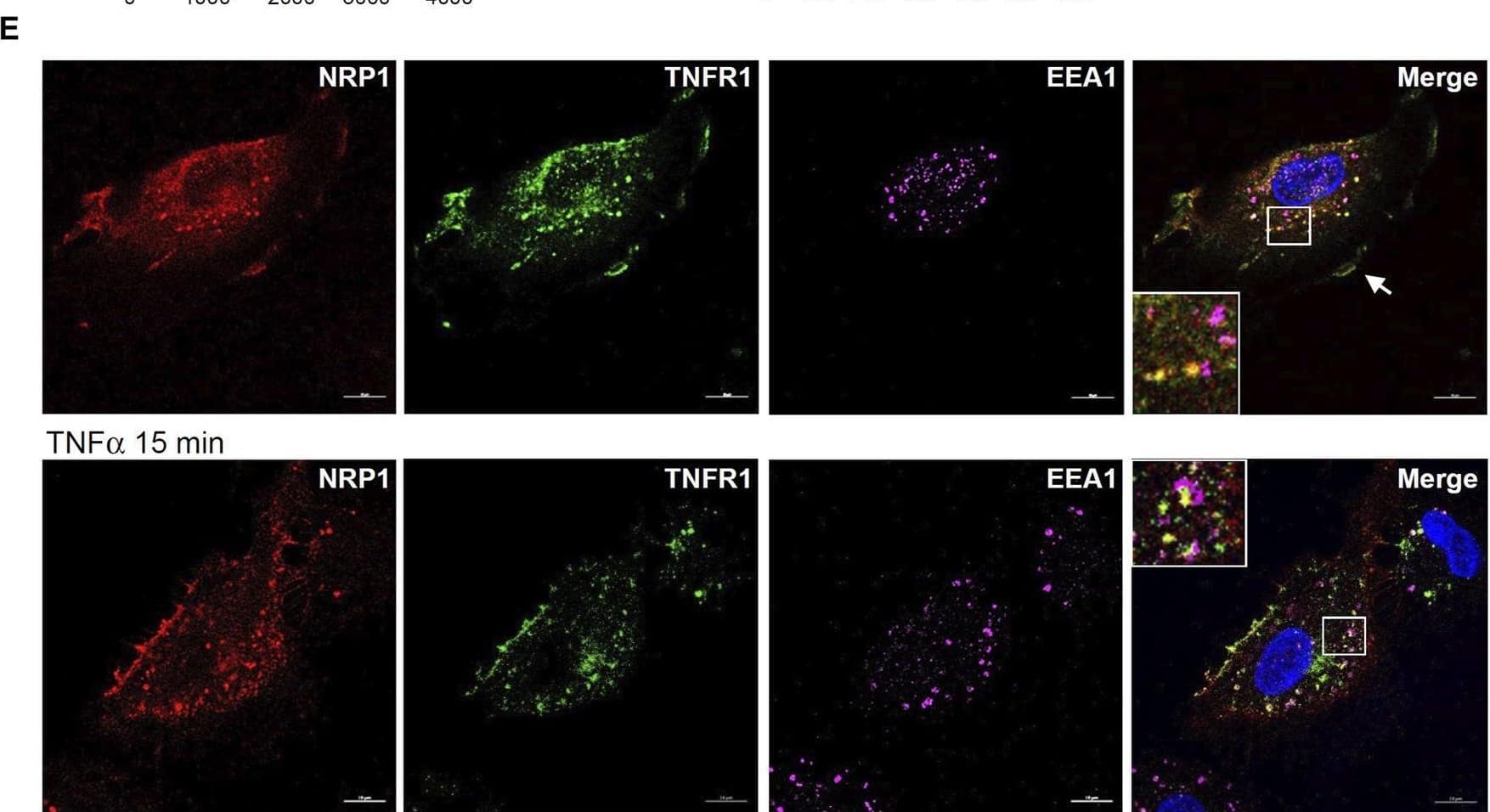
Detection of Human TNF RI/TNFRSF1A by Immunocytochemistry/ Immunofluorescence NRP1 and TNFRs are in the same protein complexes. (A) HUVECs were infected with retrovirus expressing NRP1 or LacZ as a control, and then stimulated with TNF alpha (5 ng/mL, 15 min). Cell lysates were immunoprecipated with anti-TNFR1 or TNFR2 antibodies and immunoprecipates were analyzed for the presence of the TNFRs and NRP1 by Western blotting (left panels). Cell lysates were immunoblotted as load control (right panel). (B) HUVECs were infected lentivirus expressing control-shRNA and NRP1-shRNA, respectively, selected with puromycin for 48 h, stimulated with TNF alpha and then subjected to Western blotting. (C, D) HUVECs (5*104/well) were plated into a 24-well plate. Half of the wells were pre-incubated with an excess of “non-GpL” TNF alpha (2 μg/mL) to determine the non-specific binding of Gaussia. princeps luciferase TNF alpha (GpL-TNF alpha ). Then all the wells were incubated with GpL-TNF alpha at different concentrations for 30 min at 37°C. The non-specific binding values of the “non-GpL” TNF alpha groups were subtracted from the corresponding total binding values. The data were analyzed by a non-linear regression to a single site using the GraphPad Prism 5 software. The experiments were performed in duplicates and independently repeated for 3 times. (E) HUVECs were stimulated with TNF alpha (5 ng/mL) for 15 min and then subjected to immunofluorescent staining with the indicated antibodies. Nuclei were counterstained with DAPI. Co-localization of NRP1 and TNFR1 in both plasmal membrane (arrow) and cytoplasm were observed. Scale bar, 10 μm. Image collected and cropped by CiteAb from the following open publication (https://www.frontiersin.org/articles/10.3389/fcell.2024.1210944/full), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: TNF RI/TNFRSF1A
TNF RI occurs both in membrane bound and soluble forms and functions as a receptor for TNF-alpha and TNF-beta. In the superfamily nomenclature, it is designated TNFRSF1A.
Product Datasheets
Citations for Human TNF RI/TNFRSF1A Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
14
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
EGFR inhibits TNF-?-mediated pathway by phosphorylating TNFR1 at tyrosine 360 and 401
Authors: Nam, YW;Shin, JH;Kim, S;Hwang, CH;Lee, CS;Hwang, G;Kim, HR;Roe, JS;Song, J;
Cell death and differentiation
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
APOL1 promotes endothelial cell activation beyond the glomerulus
Authors: Miguel Carracedo, Elke Ericson, Rasmus Ågren, Anna Forslöw, Katja Madeyski-Bengtson, Anna Svensson et al.
iScience
-
Structural insights into the disruption of TNF-TNFR1 signalling by small molecules stabilising a distorted TNF
Authors: D McMillan, C Martinez-F, J Porter, D Fox, R Davis, P Mori, T Ceska, B Carrington, A Lawson, T Bourne, J O'Connell
Nature Communications, 2021-01-25;12(1):582.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Small molecules that inhibit TNF signalling by stabilising an asymmetric form of the trimer
Authors: J O'Connell, J Porter, B Kroeplien, T Norman, S Rapecki, R Davis, D McMillan, T Arakaki, A Burgin, D Fox Iii, T Ceska, F Lecomte, A Maloney, A Vugler, B Carrington, BP Cossins, T Bourne, A Lawson
Nat Commun, 2019-12-19;10(1):5795.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
CAR T Cells Targeting MISIIR for the Treatment of Ovarian Cancer and Other Gynecologic Malignancies
Authors: A Rodriguez-, P Sharma, M Poussin, AC Boesteanu, NG Minutolo, SB Gitto, DK Omran, MK Robinson, GP Adams, F Simpkins, DJ Powell
Mol. Ther., 2019-12-06;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
NKp46+ Innate Lymphoid Cells Dampen Vaginal CD8 T Cell Responses following Local Immunization with a Cholera Toxin-Based Vaccine.
Authors: Luci C, Bekri S, Bihl F, Pini J, Bourdely P, Nouhen K, Malgogne A, Walzer T, Braud V, Anjuere F
PLoS ONE, 2015-12-02;10(12):e0143224.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC-Fr -
Aspirin Action in Endothelial Cells: Different Patterns of Response Between Chemokine CX3CL1/CX3CR1 and TNF-alpha /TNFR1 Signaling Pathways
Authors: Dariusz Szukiewicz, Malgorzata Wojciechowska, Anna Bilska, Aleksandra Stangret, Grzegorz Szewczyk, Tarun Kumar Mittal et al.
Cardiovascular Drugs and Therapy
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Ubiquitin-associated domain-containing ubiquitin regulatory X (UBX) protein UBXN1 is a negative regulator of nuclear factor kappaB (NF-kappaB) signaling.
Authors: Wang Y, Tan B, Mu R, Chang Y, Wu M, Tu H, Zhang Y, Guo S, Qin X, Li T, Li W, Li A, Zhang X, Li H
J Biol Chem, 2015-02-13;290(16):10395-405.
Species: Human
Sample Types: Whole Cells
Applications: Immunoprecipitation -
EI24 regulates epithelial-to-mesenchymal transition and tumor progression by suppressing TRAF2-mediated NF-kappa B activity
Authors: Jung-Min Choi, Sushil Devkota, Young Hoon Sung, Han-Woong Lee
Oncotarget
-
Pellino3 targets RIP1 and regulates the pro-apoptotic effects of TNF-alpha
Authors: Shuo Yang, Bingwei Wang, Lisa S. Tang, Jakub Siednienko, John J. Callanan, Paul N. Moynagh
Nature Communications
-
Involvement of the Same TNFR1 Residue in Mendelian and Multifactorial Inflammatory Disorders
Authors: Isabelle Jéru, Serge Charmion, Emmanuelle Cochet, Bruno Copin, Philippe Duquesnoy, Maria Teresa Mitjavila Garcia et al.
PLoS ONE
-
Pre-analytical effects of blood sampling and handling in quantitative immunoassays for rheumatoid arthritis.
Authors: Zhao X, Qureshi F, Eastman PS, Manning WC, Alexander C, Robinson WH, Hesterberg LK
J. Immunol. Methods, 2012-02-17;378(1):72-80.
Species: Human
Sample Types: Serum
Applications: ELISA Development -
Inflammatory cytokines stimulate the adhesion of colon carcinoma cells to mesothelial monolayers.
Authors: van Grevenstein WM, Hofland LJ, van Rossen ME, van Koetsveld PM, Jeekel J, van Eijck CH
Dig. Dis. Sci., 2007-03-30;52(10):2775-83.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Stent implantation in coronary porcine arteries is associated with early activation of TNFalpha and TNFalpha receptor II expression.
Authors: Hadjadj S, Cloutier I, Geoffroy P, Tanguay JF
Atherosclerosis, 2006-07-20;192(1):25-32.
Species: Porcine
Sample Types: Whole Tissue
Applications: IHC-P
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human TNF RI/TNFRSF1A Antibody
There are currently no reviews for this product. Be the first to review Human TNF RI/TNFRSF1A Antibody and earn rewards!
Have you used Human TNF RI/TNFRSF1A Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image




