Recombinant Human IL-4 GMP Protein, CF Summary
Product Specifications
The specific activity of recombinant human IL-4 is >1.0 x 107 IU/mg, which is calibrated against the human IL-4 WHO International Standard (NIBSC code: 88/656).
His25-Ser153, with an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.
Manufactured and tested under cGMP guidelines.
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
204-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 100-200 μg/mL in PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
GMP-grade Recombinant Human IL‑4 (Catalog # 204‑GMP) stimulates proliferation of TF‑1 human erythroleukemic cells. The ED50 is 0.05‑0.2 ng/mL.
 View Larger
View Larger
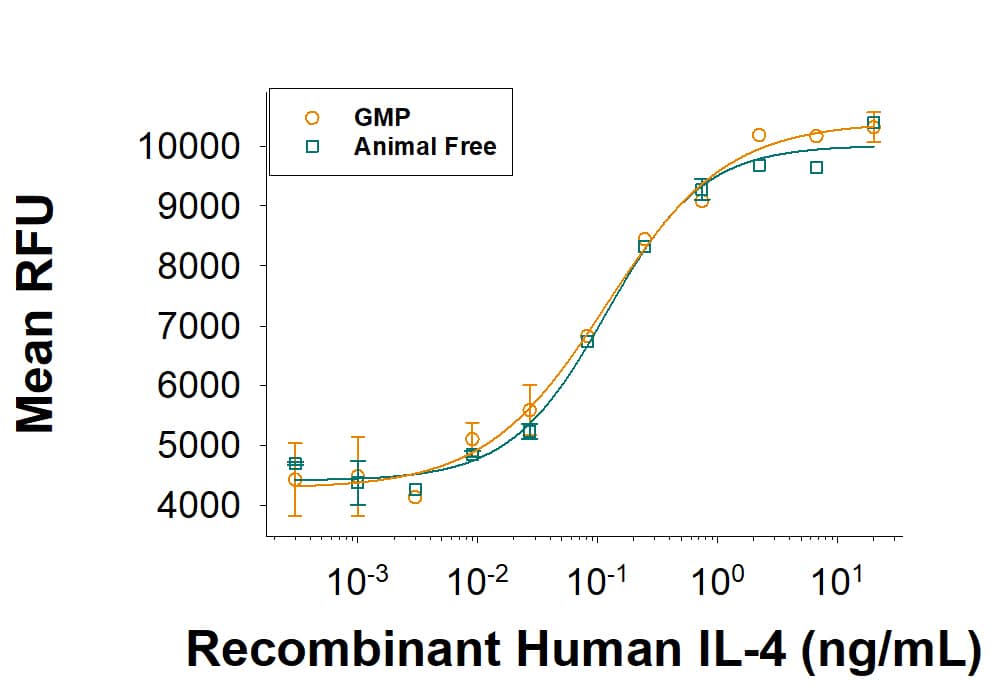
Equivalent bioactivity of GMP (Catalog # 204-GMP) and Animal-Free (AFL204) grades of Recombinant Human IL-6 as measured in cell proliferation assay (orange and green respectively).
 View Larger
View Larger
1 μg/lane of GMP-grade Recombinant Human IL-4 (Catalog # 204-GMP) was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 14 kDa.
 View Larger
View Larger
MALDI-TOF analysis of GMP-grade Recombinant Human IL-4 (Catalog # 204-GMP). The major peak at 15083 Da corresponds to the calculated molecular mass, 15094 Da. The minor peak at 15297 Da is a matrix-associated artifact of the MALDI-TOF.
Reconstitution Calculator
Background: IL-4
Interleukin-4 (IL-4), also known as B cell-stimulatory factor-1, is a monomeric, approximately 13 kDa‑18 kDa Th2 cytokine that shows pleiotropic effects during immune responses (1‑3). It is a glycosylated polypeptide that contains three intrachain disulfide bridges and adopts a bundled four alpha -helix structure (4). Human IL-4 is synthesized with a 24 aa signal sequence. Alternate splicing generates an isoform with a 16 aa internal deletion. Mature human IL-4 shares 55%, 39% and 43% aa sequence identity with bovine, mouse, and rat IL-4, respectively. Human, mouse, and rat IL-4 are species-specific in their activities (5‑7). IL-4 exerts its effects through two receptor complexes (8, 9). The type I receptor, which is expressed on hematopoietic cells, is a heterodimer of the ligand binding IL-4 R alpha and the common gamma chain (a shared subunit of the receptors for IL-2, -7, -9, -15, and ‑21). The type II receptor on nonhematopoietic cells consists of IL-4 R alpha and IL‑13 R alpha 1. The type II receptor also transduces IL-13 mediated signals. IL-4 is primarily expressed by Th2-biased CD4+ T cells, mast cells, basophils, and eosinophils (1, 2). It promotes cell proliferation, survival, and immunoglobulin class switch to IgG4 and IgE in human B cells, acquisition of the Th2 phenotype by naïve CD4+ T cells, priming and chemotaxis of mast cells, eosinophils, and basophils, and the proliferation and activation of epithelial cells (10‑13). IL-4 plays a dominant role in the development of allergic inflammation and asthma (12, 14).
- Benczik, M. and S.L. Gaffen (2004) Immunol. Invest. 33:109.
- Chomarat, P. and J. Banchereau (1998) Int. Rev. Immunol. 17:1.
- Yokota, T. et al. (1986) Proc. Natl. Acad. Sci. 83:5894.
- Redfield, C. et al. (1991) Biochemistry 30:11029.
- Ramirez, F. et al. (1988) J. Immunol. Meth. 221:141.
- Leitenberg, D. and T.L. Feldbush (1988) Cell. Immunol. 111:451.
- Mosman, T.R. et al. (1987) J. Immunol. 138:1813.
- Mueller, T.D. et al. (2002) Biochim. Biophys. Acta 1592:237.
- Nelms, K. et al. (1999) Annu. Rev. Immunol. 17:701.
- Paludan, S.R. (1998) Scand. J. Immunol. 48:459.
- Corthay, A. (2006) Scand. J. Immunol. 64:93.
- Ryan, J.J. et al. (2007) Crit. Rev. Immunol. 27:15.
- Grone, A. (2002) Vet. Immunol. Immunopathol. 88:1.
- Rosenberg, H.F. et al. (2007) J. Allergy Clin. Immunol. 119:1303.
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers.
Product Specific Notices
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.Citations for Recombinant Human IL-4 GMP Protein, CF
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
2
Citations: Showing 1 - 2
Filter your results:
Filter by:
-
Autologous HIV-specific T cell therapy targeting conserved epitopes is well-tolerated in six adults with HIV: an open-label, single-arm phase 1 study
Authors: Sohai, DK;Keller, MD;Hanley, PJ;Hoq, F;Kukadiya, D;Datar, A;Reynolds, E;Copertino, DC;Lazarski, C;McCann, CD;Tanna, J;Shibli, A;Lang, H;Zhang, A;Chansky, PA;Motta, C;Huynh, TT;Dwyer, B;Wilson, A;Lynch, R;Mota, TM;Conce Alberto, WD;Brumme, ZL;Kinloch, NN;Cruz, CRY;MacLaren Ehui, L;Henn, S;Brad Jones, R;Bollard, CM;
Nature communications
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Antiviral cellular therapy for enhancing T-cell reconstitution before or after hematopoietic stem cell transplantation (ACES): a two-arm, open label phase II interventional trial of pediatric patients with risk factor assessment
Authors: Keller, MD;Hanley, PJ;Chi, YY;Aguayo-Hiraldo, P;Dvorak, CC;Verneris, MR;Kohn, DB;Pai, SY;Dávila Saldaña, BJ;Hanisch, B;Quigg, TC;Adams, RH;Dahlberg, A;Chandrakasan, S;Hasan, H;Malvar, J;Jensen-Wachspress, MA;Lazarski, CA;Sani, G;Idso, JM;Lang, H;Chansky, P;McCann, CD;Tanna, J;Abraham, AA;Webb, JL;Shibli, A;Keating, AK;Satwani, P;Muranski, P;Hall, E;Eckrich, MJ;Shereck, E;Miller, H;Mamcarz, E;Agarwal, R;De Oliveira, SN;Vander Lugt, MT;Ebens, CL;Aquino, VM;Bednarski, JJ;Chu, J;Parikh, S;Whangbo, J;Lionakis, M;Zambidis, ET;Gourdine, E;Bollard, CM;Pulsipher, MA;
Nature communications
Species: Human
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human IL-4 GMP Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant Human IL-4 GMP Protein, CF and earn rewards!
Have you used Recombinant Human IL-4 GMP Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image







