Recombinant Human IL-7 GMP Protein, CF
New! Bypass reconstitution steps by using a liquid formulation of GMP-grade Recombinant Human IL-7. Find out more here.
Recombinant Human IL-7 GMP Protein, CF Summary
- Single use vials supplement 1L of T Cell Media (Cat # CCM038-GMP) to support T Cell Cultures in a G-Rex 100M bioreactor
- Liquid formulation minimizes reconstitution steps
- Lot-to-lot consistency
- Stringent guidelines for patient safety
- Scalability necessary to support successful therapeutics
- Learn more about manufacturing in our new GMP facility
- Test it in your process! Request a sample of GMP IL-7
Product Specifications
Asp26-His177, with an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.
Manufactured and tested under cGMP guidelines.
Analysis
Product Datasheets
BT-007-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 100-500 μg/mL in PBS. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
BT-007-GMP/LQ
| Formulation | Supplied as a 0.2 μm filtered solution in PBS. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
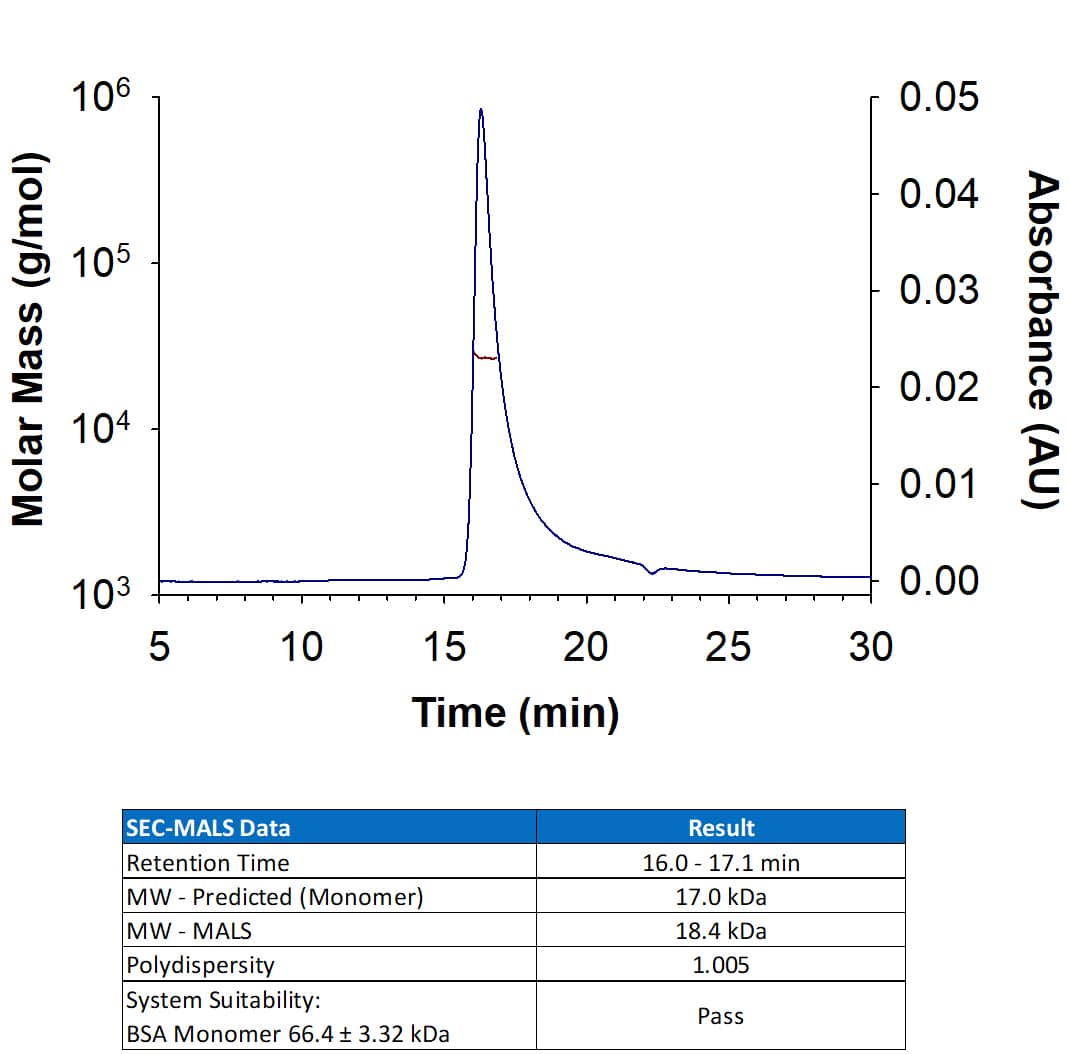
Recombinant Human IL-7 GMP (Catalog # BT-007-GMP) has a molecular weight (MW) of 18.4 kDa as analyzed by SEC-MALS, suggesting that this protein is a monomer.
 View Larger
View Larger
GMP-grade Recombinant Human IL-7 (Catalog # BT-007-GMP) stimulates proliferation of PHA-activated human peripheral blood lymphocytes. The ED50 for this effect is 0.100-0.500 ng/mL. Three independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of GMP IL-7.
 View Larger
View Larger
2 μg/lane of GMP-grade Recombinant Human IL-7 Protein (Catalog # BT-007-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing a single band at 17 kDa.
 View Larger
View Larger
Analysis of GMP-grade Recombinant Human IL-7 (Catalog # BT-007-GMP), showing a major peak at 17501 Da.
 View Larger
View Larger
Four samples of GMP-grade human IL-7 (Catalog number BT-007-GMP, green circles) were interpolated with the human IL-7 Quantikine High Sensitivity ELISA Kit (HS750) standard curve (black circles) using 4PL logistic regression analysis. The IL-7 Quantikine High Sensitivity ELISA Kit has an assay range of 0.156-10 pg/mL for cell culture supernates and 0.25-16 pg/mL for serum, EDTA plasma and citrate plasma.
 View Larger
View Larger
Thirteen samples of GMP-grade human IL-7 (Catalog number BT-007-GMP, green circles) were interpolated with the Simple Plex Human IL-7 cartridge (SPCKB-PS-000506) standard curve (black circles) using 4PL logistic regression analysis. The Simple Plex IL-7 cartridge has an assay range of 0.57-2,170 pg/mL.
 View Larger
View Larger
Three independent lots of Recombinant Human IL-7 GMP Protein (Catalog # BT-007-GMP) were analyzed by Maurice CE-SDS PLUS (IS is an Internal Standard). A gel-like representation of the purity analysis data (inset) can be obtained from the Lane View feature in Compass software for iCE. Profiles from the three runs were superimposed, showing excellent manufacturing consistency.
 View Larger
View Larger
Three independent lots of Recombinant Human IL-7 GMP Protein (Catalog # BT-007-GMP) were analyzed by Maurice icIEF using native fluorescence detection (Mkr 5.85 and 9.99 are pI Markers). Profiles from the three runs were superimposed, showing excellent manufacturing consistency.
 View Larger
View Larger
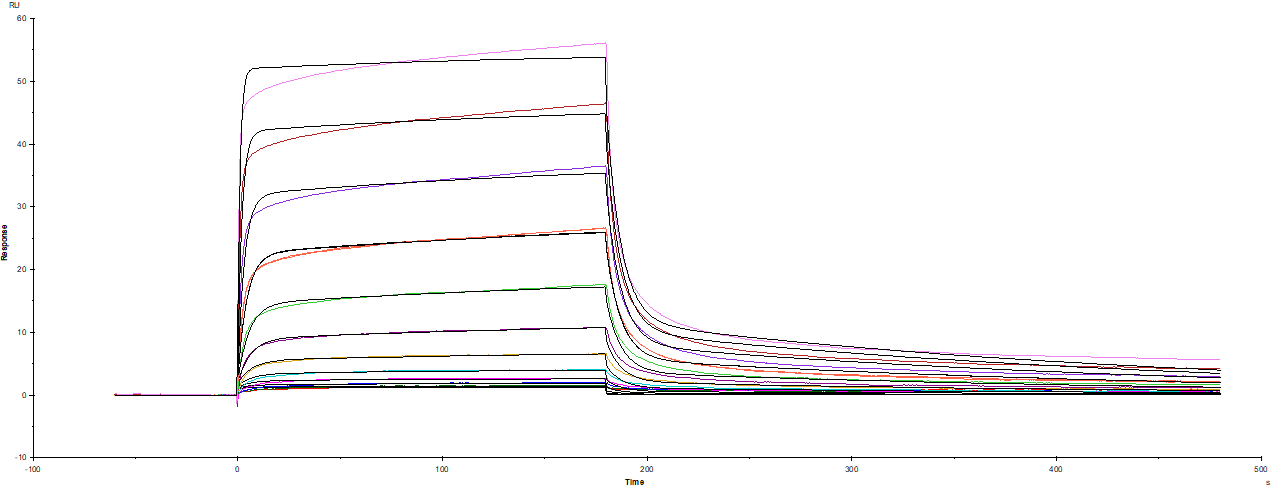
Recombinant Human IL-7R alpha/CD127 Fc Chimera (306-IR) was captured on Biacore Sensor Chip CM5 via Recombinant Protein A/G/L (Catalog # NBP2-34985), and binding to Recombinant Human IL-7 GMP (Catalog # BT-007-GMP) was measured at a concentration range between 0.244 nM and 500 nM. The double-referenced sensorgram was fit to a 1:1 binding model to determine the binding kinetics and affinity, with an affinity constant of KD=62.6 nM. (Biacore T200).
Reconstitution Calculator
Background: IL-7
IL-7 (interleukin-7) is a 25 kDa cytokine of the hemopoietin family that plays important roles in lymphocyte differentiation, proliferation, and survival (1-4). Human IL‑7 cDNA encodes 177 amino acids (aa) that include a 25 aa signal peptide (3). Human IL-7 shares approximately 60-63% aa sequence identity with mouse, rat, canine and feline IL-7, and 72-76% with equine, bovine, ovine, and porcine IL-7. Human and mouse IL-7 exhibit cross-species activity (2, 3).
- Sasson, S.C. et al. (2006) Curr. Drug Targets 7:1571.
- Barata, J.T. et al. (2006) Exp. Hematol. 34:1133.
- Goodwin, R.G. et al. (1990) Proc. Natl. Acad. Sci. USA 86:302.
- Namen, A.E. et al. (1988) Nature 333:571.
- Shalapour, S. et al. (2012) PLoS ONE 7: e31939.
- Saini, M. et al. (2009) Blood 113:5793.
- Park, J.H. et al. (2004) Immunity 21:289.
- Vranjkovic, A. et al. (2007) Int. Immunol. 19:1329.
- Sudo, T. et al. (1993) Proc. Natl. Acad. Sci. 90:9125.
- Seddon, B. et al. (2003) Nat. Immunol. 4:680.
- Schluns, K.S. et al. (2000) Nat. Immunol. 5:426.
- Peschon, J.J. et al. (1994) J. Exp. Med. 180:1955.
- Pribyl, J.A. and T.W. LeBien (1996) Proc. Natl. Acad. Sci. 93:10348.
- Johnson, K. et al. (2012) J. Immunol. 188:6084.
- Nodland, S.E. et al. (2011) Blood 118:2116.
- Wang, C. et al. (2022) Int. J. Mol. Sci. 23:10370.
- Pang, N. et al. (2021) J Hematol Oncol. 14:118.
- Li, L. et al. (2022) Sci Rep. 12:12506.
- Xu, Y. et al. (2014) Blood. 123:3750.
- Marton,C. et al. (2022) Cancer Gene Ther. 29:961.
- Winer, H. et al. (2022) Cytokine. 160:156049.
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers.
Product Specific Notices
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human IL-7 GMP Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant Human IL-7 GMP Protein, CF and earn rewards!
Have you used Recombinant Human IL-7 GMP Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image




