GMP Proteins For Cell Therapy Manufacturing
Develop Your Cell Therapy Manufacturing Process With Confidence
Using R&D Systems GMP Cytokines and Growth Factors
Highly consistent GMP proteins are key ancillary reagents for building your cell manufacturing program. We follow all relevant regulatory guidelines to ensure a consistent, safe, and traceable supply of animal-free bioactive proteins.
Years of Protein Manufacturing Expertise
Leveraging years of expertise in protein development, manufacturing, quality control, and regulatory support, we offer industry-leading GMP proteins for ancillary use.
Seamless Transition From Preclinical to GMP
We ensure a seamless transition to GMP by using the same clone, sequence, and expression system as our RUO and animal-free grade materials.
Reliable Supplier
As cellular therapies advance, the demand for high-quality raw materials like GMP cytokines and growth factors grows. Our large, consistent, safe, and traceable supply of GMP proteins supports cell therapy.
Compliance Documentation
Recognizing customers’ regulatory filing end goals, many of our GMP proteins are supported by drug master files, facilitating your IND's swift approval. Certificates of Analysis are available for released lots.
Dedicated Custom Team
Sometimes cell therapies need tailor-made protein options. For custom GMP proteins or formulations, our dedicated team will work with you to provide personalized fill options.
Available GMP Proteins
ǂ DMF is on file
ProPak™ GMP Cytokines: For Closed Process Manufacturing
GMP IL-2, IL-7, and IL-15 are now available in single-use weldable bags. These 1 L process-sized protein bags reduce both variability and risk for your cell therapy, plus eliminate reconstitution and aliquoting.

Lot-to-Lot Consistency
As you scale your clinical manufacturing process, it often becomes necessary to use GMP cytokines and growth factors from different lots. Through decades of manufacturing expertise, R&D Systems had developed a mature quality management system with detailed protocols, creating recombinant proteins with industry-leading consistency. Each new lot is tested against a master lot and must pass stringent quality control specifications for activity in a well-defined bioassay.

Three independent lots of GMP IL-2 (A, Catalog # BT-002-GMP), IL-7 (B, Catalog # BT-007-GMP), and IL-10 (C, Catalog # 1064-GMP) were tested for their ability to stimulate proliferation of CTLL-2 mouse cytotoxic T cells, PHA-activated human peripheral blood lymphocytes, and MC/9-2 mast cells, respectively. Each trace on the graphs represents data obtained from a different manufacturing run, demonstrating the lot-to-lot consistency of each protein.
Grade-to-Grade Consistency
Not only are the proteins consistent lot-to-lot, but also across RUO, animal-free RUO, and GMP grades. Both animal-free RUO and GMP grades of E. coli sourced proteins share the same sequence, source, formulation, activity and purity specifications, manufacturing site and personnel, and vial type – ensuring equivalent performance and process continuity as you transition to the clinic.
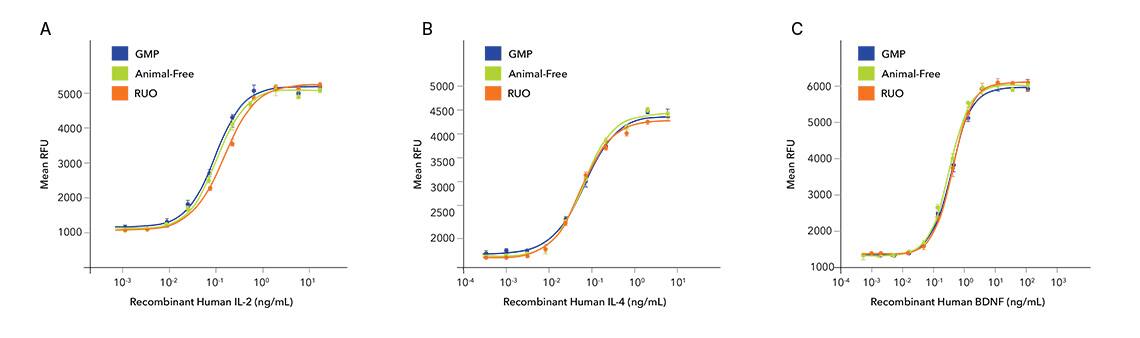
The three grades of IL-2 (A, Catalog # BT-002, BT-002-AFL, and BT-002-GMP), IL-4 (B, Catalog # BT-004, BT-004-AFL, and BT-004-GMP), and BDNF (C, Catalog # BT-BDNF, BT-BDNF-AFL, and BT-BDNF-GMP) were tested for their ability to stimulate proliferation of CTLL-2 mouse cytotoxic T cells, TF-1 human erythroleukemic cells, and BaF mouse pro-B cells transfected with TrkB, respectively. All grades show equivalent bioactivity, enabling a seamless transition as higher levels of regulation and documentation are needed.
GMP Quality Policy and Regulatory Support
GMP-grade cytokines and growth factors are produced under regulatory guidelines for ancillary materials in cell therapy manufacturing processes. This includes extensive quality control testing and comprehensive documentation of manufacturing systems and traceability of source materials. You can be confident you will receive a consistent, safe, and traceable supply of raw materials. We perform regular audits of our facilities and welcome customer audits.
We're committed to meeting the increasing demands of therapeutic cell manufacturing. Our animal-free facility in St. Paul, Minnesota supports large-scale production of GMP-grade, E. coli-derived recombinant proteins.
- Entire facility dedicated to manufacturing GMP proteins
- Completely animal-free – No animal components are allowed in the facility
- Capacity – 61,000 sq. ft. to meet your current and future supply requirements
- Expandability – Available space to build additional capacity
- ISO 5/7/8 cleanrooms for the entire production process
- ISO 9001:2015, ISO 13485:2016-certified facilities including a completely animal-free facility dedicated to manufacturing GMP proteins
- USP Chapter <1043>, Ancillary Materials for Cell, Gene, and Tissue-Engineered Products
- Ph. Eur. General Chapter 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products
We work with preferred vendors for raw materials to mitigate risk and adhere to a strict definition of animal-free conditions. Certificates of Origin (COOs) are available upon request.
- Individual specification sheets with all testing results, reviewed by both our Quality Control (QC) and Quality Assurance (QA) departments
- Lot-specific Certificates of Analysis
- Full QA review of all batch and bottling records before any material is shipped
- Documented processes and QA control of documentation and process changes
- Personnel training programs
- Raw material testing, tracing, and vendor qualification/monitoring
- Fully validated equipment, processes, and test methods
- Equipment calibration schedules using a computerized calibration program
- Facility maintenance and safety programs
- Material review board for variances
- Stability monitoring over the shelf life of each product
- Product change notifications
We pay strict attention to detail at all levels of the protein manufacturing process. Our quality management systems for GMP proteins include detailed Standard Operating Procedures (SOPs) and quality-controlled documentation of the manufacturing equipment and procedures. You can rely on our GMP proteins for building a consistent and robust cell therapy manufacturing program.
- Biological potency specification
- Defined purity specifications
- Stability testing program
- Defined endotoxin specifications
- Host cell protein content testing
- Sterility testing according to USP
- Additional testing available depending on your needs
We provide each manufactured lot of GMP protein with a Certificate of Analysis (CofA) that documents the relevant quality systems and product specifications. Prior to CofA release, we perform a full QA review of all batch and bottling records. Here is what you can expect to find on our CofAs:
- Actual vial fill (protein mass) for accurate reconstitution
- Source information
- N-terminal sequencing of the first 10 amino acids
- Purity specification
- Bioactivity assay including benchmarking against a master lot
- Sterility testing to USP
- Endotoxin levels
- Host cell protein specification
- Host cell DNA specification
- Stability statement
- Certifications and regulatory guidelines followed
