Transform Your Cell Therapy Manufacturing With Non-Viral Gene Delivery
Fast, Scalable, and Cost-Effective Stable Gene Transfer Solution
TcBuster is a large capacity transposon system that enables transfer and stable integration of multiple genes of interest. An effective alternative to viral vectors, TcBuster offers significant time and cost savings during the development of cell therapies, such as CAR T or CAR NK.
TcBuster Reagents and Uses
| mRNA Transposase | TCB-001.1-100 (100 µg) | 100 tests at 1 µg each | - Useful for system evaluation |
| TCB-001.1-500 (500 µg) | 500 tests at 1 µg each | - Useful for further development | |
| TCB-001.1-GMP (500 µg) | 500 tests at 1 µg each | - GMP grade - Useful for pre-IND process development and ex vivo clinical applications | |
| TcBuster-Compatible Transposons | TCBP001-100 (100 µg) | 25-100 tests at 1-4 µg each | - Multicistronic CAR-containing transposon - Useful in cell therapy applications as an experimental control |
| TCBP002-100 (100 µg) | 25-100 tests at 1-4 µg each | - Whole plasmid insertion of eGFP with no untransposed plasmid expression - Useful in bioprocessing system evaluation as an experimental control | |
| Custom Transposons | Contact techsupport@bio-techne.com for more information | - For process development using your own therapeutic cargo - Utilizes partnership with Aldevron™ | |
| Anti-TcBuster Transposase Antibody | MAB11511 (25 µg, 100 µg) | 1 µg/mL for Western Blot 20 µg/mL for Simple Western | - Primary antibody detects TcBuster transposase enzyme in Western blotting applications |
How Does the TcBuster System Compare to Virus-Based Systems?
Gene transfer is a critical step in generating cell therapies and biologics. While viral vector-based engineering (e.g. lentivirus or AAV) has been the standard for delivering DNA cargo to cells, the TcBuster system provides an alternative, non-viral solution using standard electroporation methods. This system has several advantages over traditional viral options:
- Reduced time to market and cost requirements for cell therapies
- Increased cargo capacity, high editing efficiency, and delivery of multiple genes in single vector
- Stable expression of DNA cargo with a de-risked insertional profile
- Strong and reliable supply chain for both RUO & GMP grade material
- Scale effortlessly from research to clinical and commercial stages with reduced batch-to-batch variability and no need for viral preps
- Flexible and non-exclusive license options plus robust technical and regulatory support
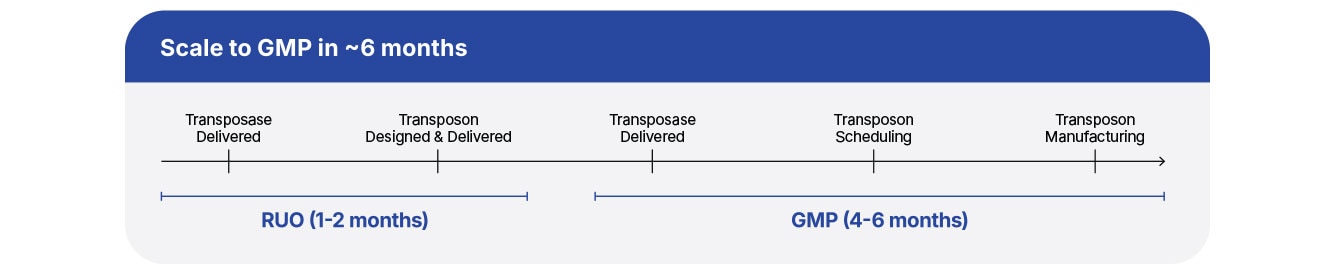
Saving Time Means Saving Money
Get Your Therapy to Market Faster

A Story of Success: Luminary Therapeutics
Luminary Therapeutics is using the TcBuster system to manufacture their cell therapies, and their LMY-920 program has passed 3 INDs with TcBuster! At SITC 2024, Phase 1 results were reported for a BAFF CAR-T trial using TcBuster, demonstrating the first successful clinical use of the system.
"Moreover, the novel, clinical-stage TcBuster transposon system is utilized to improve manufacturing timelines, efficiency, cost, and safety."
