Cycloheximide
Tocris Bioscience | Catalog # 0970

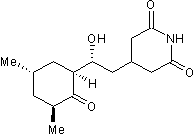
Key Product Details
Description
Product Description
Cycloheximide is a selective inhibitor of eukaryotic (over prokaryotic) protein synthesis, blocking tRNA binding and release from ribosomes. Induces apoptosis in a variety of transformed and normal cell lines, including T cells. Competitively inhibits the PPIase hFKBP12 (Ki = 3.4 μM). Also inhibits ferroptosis. Antifungal antibiotic. Exhibits anti-MERS-CoV activity in Vero cells in vitro (IC50 = 0.16 μM).
Product Specifications for Cycloheximide
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| water | 7.03 | 25 | |
| Ethanol | 14.07 | 50 |
Preparing Stock Solutions for Cycloheximide
The following data is based on the product molecular weight 281.35.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 0.5 mM | 7.11 mL | 35.54 mL | 71.09 mL |
| 2.5 mM | 1.42 mL | 7.11 mL | 14.22 mL |
| 5 mM | 0.71 mL | 3.55 mL | 7.11 mL |
| 25 mM | 0.14 mL | 0.71 mL | 1.42 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 166 publications citing the usage of this product.
- Sawicki and Sawicki Coronavirus minus-strand RNA synthesis and effect of cycloheximide on coronavirus RNA synthesis. J.Virol. 1986 PMID: 2867230
- Ko Screening of FDA-approved drugs using a MERS-CoV clinical isolate from South Korea identifies potential therapeutic options for COVID-19. Viruses 2021 PMID: 33918958
- Xie Ferroptosis: process and function. Cell.Death.Differ. 2016 PMID: 26794443
- Tang Cycloheximide-induced T-cell death is mediated by a Fas-associated death domain-dependent mechanism. J.Biol.Chem. 1999 PMID: 10066786
- Obrig The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J.Biol.Chem. 1971 PMID: 5541758
- Christner Synthesis and cytotoxic evaluation of cycloheximide derivatives as potential inhibitors of FKBP12 with neuroregenerative properties. J.Med.Chem. 1999 PMID: 10479292
Product Documents for Cycloheximide
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Cycloheximide
For research use only
⚠ WARNING: This product can expose you to chemicals including Cycloheximide, which is known to the State of California to cause reproductive toxicity with developmental effects. For more information, go to www.P65Warnings.ca.govCitations for Cycloheximide
Customer Reviews for Cycloheximide (3)
Have you used Cycloheximide?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Species: RatAssay Type: In VitroCell Line/Tissue: H9C2Verified Customer | Posted 05/28/2021Treated H9C2 to inhibit protein translation.
-
Species: MouseAssay Type: In VitroCell Line/Tissue: AML12Verified Customer | Posted 01/11/2018required dilution made fresh.20 microgram/ml for 5 hours
-
Species: HumanVerified Customer | Posted 01/04/2018Product is easy to resuspend and works well with experiments
There are no reviews that match your criteria.

