SAHA
Tocris Bioscience | Catalog # 4652

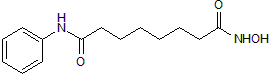
Key Product Details
Description
Alternative Names
Product Description
SAHA inhibits Class I and II histone deacetylases (HDACs); induces accumulation of acetylated histones H2A, H2B, H3 and H4 in transformed cultured cells. Suppresses cell growth in a range of cancer cell lines; induces apoptosis in cutaneous T cell lymphoma cells in vitro. Activates autophagy. SAHA increases efficiency of transcription factor-induced reprogramming of mouse embryonic fibroblasts (MEF) to induced pluripotent stem cells (iPSC). Enhances adeno-associated virus transduction of HeLa cells. Also induces activation and replication of latent HIV in vitro.
Product Specifications for SAHA
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 26.43 | 100 |
Preparing Stock Solutions for SAHA
The following data is based on the product molecular weight 264.32.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 3.78 mL | 18.92 mL | 37.83 mL |
| 5 mM | 0.76 mL | 3.78 mL | 7.57 mL |
| 10 mM | 0.38 mL | 1.89 mL | 3.78 mL |
| 50 mM | 0.08 mL | 0.38 mL | 0.76 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 34 publications citing the usage of this product.
- Nicolson Identification and validation of small molecules that enhance recombinant adeno-associated virus transduction following high-throughput screens. J.Virol. 2016 PMID: 27147738
- Narasipura Epigenetic regulation of HIV-1 latency in astrocytes. J.Virol. 2014 PMID: 24352441
- Galluzzi Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat.Rev.Drug.Discov. 2017 PMID: 28529316
- Huangfu Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat.Biotechnol. 2008 PMID: 18568017
- Marks and Breslow Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat.Biotechnol. 2007 PMID: 17211407
- Leoni The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc.Natl.Acad.Sci.U.S.A 2002 PMID: 11867742
- Butler Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000 PMID: 11016644
Product Documents for SAHA
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for SAHA
For research use only
Citations for SAHA
Customer Reviews for SAHA (1)
Have you used SAHA?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Species: RatAssay Type: In VitroCell Line/Tissue: PC12Verified Customer | Posted 11/25/2021SAHA can be easily resuspended in DMSO. Make a stock solution with a concentration that will not exceed 0.01% of DMSO in the cell culture to avoid toxicity.Our lab uses this drug to monitor transcriptional activity of genes that depend on class I, II and IV of histone deacetylase. In the experiment shown in the figure, we have treated PC12 cells with 3 different concentrations of SAHA for 3h. We found increased transcriptional activity of PI3K gene upon SAHA treatment in a dose-dependent manner.
There are no reviews that match your criteria.

