CPPG
Tocris Bioscience | Catalog # 0972

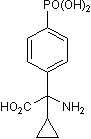
Key Product Details
Description
Product Description
CPPG is a potent group II/III mGlu receptor antagonist, with approximately 20-fold selectivity for group III over group II (IC50 values of 2.2 and 46.2 nM respectively). A much less potent antagonist at group I receptors in neonatal rat cortical slices (KB = 0.65 ± 0.07 nM).
Product Specifications for CPPG
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| 1eq. NaOH | 27.12 | 100 |
Preparing Stock Solutions for CPPG
The following data is based on the product molecular weight 271.21.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 3.69 mL | 18.44 mL | 36.87 mL |
| 5 mM | 0.74 mL | 3.69 mL | 7.37 mL |
| 10 mM | 0.37 mL | 1.84 mL | 3.69 mL |
| 50 mM | 0.07 mL | 0.37 mL | 0.74 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 111 publications citing the usage of this product.
- Toms The effects of (RS)-α-cyclopropyl-4-phosphonophenylglycine ((RS)-CPPG), a potent and selective metabotropic glutamate receptor antagonist. BrJ.Pharmacol. 1996 PMID: 8922731
- Kemp α-Methyl-3-phosphonophenylglycine and α-cyclopropyl-4-phosphonophenylglycine are potent antagonists at mGluRs negatively coupled to adenylyl cyclase. Br.J.Pharmacol.
- Jane Potent antagonists at the L-AP4- and (1S, 3S)-ACPD-sensitive presynaptic metabotropic glutamate receptors in the neonatal rat spinal cord. Neuropharmacology 1996 PMID: 9121605
Product Documents for CPPG
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for CPPG
For research use only
Citations for CPPG
Customer Reviews for CPPG (2)
Have you used CPPG?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Species: RatAssay Type: In VitroCell Line/Tissue: Retinal rod bipolar cellsVerified Customer | Posted 12/03/2019Retinal dissections were performed in HEPES-buffered extracellular solution containing glutamate inhibitor cocktail - 600 umol/L
-
Species: RatAssay Type: In VitroCell Line/Tissue: CerebellumVerified Customer | Posted 09/17/2019Compound should be sonicated when dissolving.CPPG (5 microM) was used for pharmacological isolation of GABA-A receptor activity in nucleated membrane patches. Being added to perfusion solution, CPPG blocked contaminating receptor activity in a recorded response, thus revealing clear GABA-ergic single-channel openings. Figure: single-channel openings of GABA-A receptors in a membrane patch after application of CPPG.
There are no reviews that match your criteria.


