
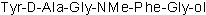
Loading...
Key Product Details
Description
Selective μ agonist
Alternative Names
DAGO
Product Description
DAMGO is a highly selective peptide agonist for the μ opioid receptor.
Product Specifications for DAMGO
Molecular Weight
513.70
Formula
C26H35N5O6
Storage
Store at -20°C
Purity
≥95% (HPLC)
Chemical Name
[D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin
CAS Number
78123-71-4
PubChem ID
5462471
InChI Key
HPZJMUBDEAMBFI-WTNAPCKOSA-N
SMILES
[H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@H](C)C(=O)NCC(=O)N(C)[C@@H](CC1=CC=CC=C1)C(=O)NCCO
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Loading...
Solubility
| Solubility | Soluble to 2mg/ml in water |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 260 publications citing the usage of this product.
- Hirning Studies in vitro with ICI 174,864, [D-Pen2,D-Pen5]-enkephalin (DPDPE) and [D-Ala2,NMePhe4,Gly-ol]-enkephalin (DAGO). Neuropeptides 1985 PMID: 2987739
- Fang Opioid receptors (DAGO-enkephalin, dynorphin A(1-13), BAM 22P) microinjected into the rat brainstem: comparison of their antinociceptive effect and their effect on neuronal firing in the rostral ventromedial medulla. Brain Res. 1989 PMID: 2572306
Product Documents for DAMGO
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for DAMGO
For research use only
Citations for DAMGO
Customer Reviews for DAMGO (2)
5 out of 5
2 Customer Ratings
Have you used DAMGO?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
Showing
1
-
2 of
2 reviews
Showing All
Filter By:
-
Species: MouseAssay Type: Ex VivoVerified Customer | Posted 03/24/2023Whole cell patch clamp recording (voltage clamp) from spinal dorsal horn interneuron, in ex vivo slice preparation, in response to bath application of DAMGO (3 µM), in the presence of TTX, bicuculline and strychnine.
-
Assay Type: In VivoCell Line/Tissue: muscleVerified Customer | Posted 12/01/2017Texting exercise presser reflex and how damgo affected contraction and blood pressure
There are no reviews that match your criteria.
Loading...

