GPBAR-A
Tocris Bioscience | Catalog # 4478

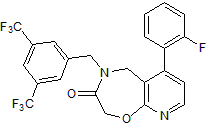
Product Description
GPBAR-A is a gPBA receptor agonist. Upregulates GLP-1 secretion from intestinal cultures. Increases MQAE-fluorescence suggesting a decrease in intracellular chloride concentration in gallbladder epithelial cells.
Product Specifications for GPBAR-A
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 48.44 | 100 | |
| Ethanol | 9.69 | 20 |
Preparing Stock Solutions for GPBAR-A
The following data is based on the product molecular weight 484.37.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 2.06 mL | 10.32 mL | 20.65 mL |
| 5 mM | 0.41 mL | 2.06 mL | 4.13 mL |
| 10 mM | 0.21 mL | 1.03 mL | 2.06 mL |
| 50 mM | 0.04 mL | 0.21 mL | 0.41 mL |
Calculators
Background References
References are publications that support the biological activity of the product.
- Keitel The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology 2009 PMID: 19582812
- Parker Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br.J.Pharmacol. 2012 PMID: 21718300
Product Documents for GPBAR-A
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for GPBAR-A
For research use only
Citations for GPBAR-A
Customer Reviews for GPBAR-A
There are currently no reviews for this product. Be the first to review GPBAR-A and earn rewards!
Have you used GPBAR-A?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
