IB-MECA
Tocris Bioscience | Catalog # 1066

Key Product Details
Description
Alternative Names
Product Description
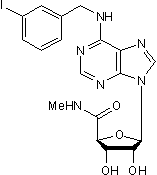
IB-MECA is a potent and selective A3 adenosine receptor agonist (Ki values are 1.1, 54 and 56 nM for A3, A1 and A2A receptors respectively). Cardioprotective, reduces infarct size upon reperfusion in rats.Licensing Information
Sold with the permission of the NIH, US Patent 08/091,109
Product Specifications for IB-MECA
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 25 |
Preparing Stock Solutions for IB-MECA
The following data is based on the product molecular weight 510.29.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 0.25 mM | 7.84 mL | 39.19 mL | 78.39 mL |
| 1.25 mM | 1.57 mL | 7.84 mL | 15.68 mL |
| 2.5 mM | 0.78 mL | 3.92 mL | 7.84 mL |
| 12.5 mM | 0.16 mL | 0.78 mL | 1.57 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 112 publications citing the usage of this product.
- Klotz Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch.Pharmacol. 2000 PMID: 11111832
- Park N6-(3-iodobenzyl)-adenosine-5'-N-methylcarboxamide confers cardioprotection at reperfusion by inhibiting mitochondrial permeability transition pore opening via glycogen synthase kinase 3β. J.Pharmacol.Exp.Ther. 2006 PMID: 16611852
- Gallo-Rodriguez Structure-activity relationships of N6-benzyladenosine-5'-uronamides as A3-selective adenosine agonists. J.Med.Chem. 1994 PMID: 8126704
Product Documents for IB-MECA
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for IB-MECA
For research use only
Citations for IB-MECA
Customer Reviews for IB-MECA
There are currently no reviews for this product. Be the first to review IB-MECA and earn rewards!
Have you used IB-MECA?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
