PD 0325901
Tocris Bioscience | Catalog # 4192

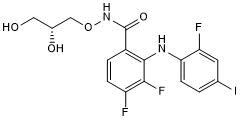
Key Product Details
Description
Alternative Names
Product Description
PD 0325901 is a potent MEK1 and MEK2 inhibitor. PD 0325901 inhibits MEK activity in mouse colon 26 cells (IC50 = 0.33 nM). PD 0325901 inhibits the growth of melanoma cell lines in vitro and in vivo; induces G1-phase cell cycle arrest and apoptosis in a mouse xenograft model. PD 0325901 also inhibits production of proangiogenic cytokines such as VEGF. PD 0325901 enhances generation of induced pluripotent stem cells (iPSCs). PD 0325901 in combination with Vitamin C sustains mouse ES cells at an undifferentiated and hypomethylated state. PD 0325901 promotes myelin recovery in drug-induced demyelination in vivo in mice model. Orally bioavailable. Used in protocols for the generation of CiPSCs from MEFs, induction of cortical neurons from hiPSCs, and culture of mESCs (see below).
For more information about how PD 0325901 may be used, see our protocols: Culturing Transcriptomically Distinct Pluripotent mESCs, Highly Efficient Generation of CiPSCs from MEFs, Accelerated Induction of Cortical Neurons from hiPSCs.
Licensing Information
Sold for research purposes under agreement from Pfizer Inc.
Product Specifications for PD 0325901
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 12.05 | 25 |
Preparing Stock Solutions for PD 0325901
The following data is based on the product molecular weight 482.19.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 0.25 mM | 8.30 mL | 41.48 mL | 82.95 mL |
| 1.25 mM | 1.66 mL | 8.30 mL | 16.59 mL |
| 2.5 mM | 0.83 mL | 4.15 mL | 8.30 mL |
| 12.5 mM | 0.17 mL | 0.83 mL | 1.66 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 352 publications citing the usage of this product.
- Li An alternative culture method to maintain genomic hypomethylation of mouse embryonic stem cells using MEK inhibitor PD0325901 and vitamin C. J.Vis.Exp. 2018 PMID: 29912180
- Suo Inhibition of MAPK/ERK pathway promotes oligodendrocytes generation and recovery of demyelinating diseases. Glia 2019 PMID: 30815939
- Koehler 3D mouse embryonic stem cell culture for generating inner ear organoids. Nat.Protoc. 2014 PMID: 24784820
- Lin A chemical platform for improved induction of human iPSCs. Nat.Methods. 2009 PMID: 19838168
- Sebolt-Leopold The biological profile of PD 0325901: a second generation analog of CI-1040 with improved pharmaceutical potential. Proc.Amer.Assoc.Cancer Res.
- Ciuffreda Growth-inhibitor and antiangiogenic activity of the MEK inhibitor PD0325901 in malignant melanoma with or without BRAF mutations. Neoplasia 2009 PMID: 19649202
- Barrett The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorg.Med.Chem.Lett. 2008 PMID: 18952427
Product Documents for PD 0325901
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for PD 0325901
For research use only
Related Research Areas
Citations for PD 0325901
Customer Reviews for PD 0325901 (8)
Have you used PD 0325901?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Species: MouseAssay Type: In VitroVerified Customer | Posted 05/08/2025Good MEKi for mESCs culture using 2i LIF media
-
Species: MouseAssay Type: In VitroCell Line/Tissue: ipsVerified Customer | Posted 09/27/2022Mouse ips cells culture with 2i media.
-
Species: MouseAssay Type: In VitroCell Line/Tissue: ESCVerified Customer | Posted 07/11/2022ESC culture using 2i medium. 1uM
-
Species: MouseAssay Type: In VitroCell Line/Tissue: ESCVerified Customer | Posted 11/23/2021In vitro cell culture addition. The image shows the morphology of ESC colonies after conversion by using the treatment condition. This condition supported the conversion and long-term maintenance of dome-shaped ESCs from primed ESCs.
-
Species: MouseAssay Type: In VitroCell Line/Tissue: ipscVerified Customer | Posted 12/04/2019All iPSC lines and R1 mouse ES cells were maintained on feeders in KO-DMEM (Invitrogen) with 3 mM CHIR99021, and 1 mM PD0325901.
-
Species: MouseAssay Type: In VivoVerified Customer | Posted 08/20/2019Used to grow mES cells in 2i conidtions. The cells grew well and I did QPCR on isolated RNA
-
Species: MouseAssay Type: In VitroCell Line/Tissue: E14TG2aVerified Customer | Posted 07/18/2018Mouse ES cells in 2i remain to be in the naive pluripotent state while cells in -2i for 48 hours exit naive pluripotency
-
Species: MouseAssay Type: In VitroCell Line/Tissue: Primary mouse lung endothelial cellsVerified Customer | Posted 12/08/201724 hours later, cell was lyzed and analyzed by western blot. Lane 1: PBS control Lane 2: + PD 0326901 100nM Lane 3: + PD 0326901 10nMPrimary lung endothelial cells were pretreated with various concentrations of PD 0325901 for 30 minutes prior to the treatment of IL1b (1ng/ml).
There are no reviews that match your criteria.








