Tomoxetine hydrochloride
Tocris Bioscience | Catalog # 2011

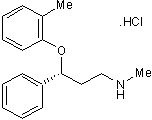
Key Product Details
Description
Alternative Names
Product Description
Tomoxetine hydrochloride is a potent and selective noradrenalin re-uptake inhibitor (Ki values are 5, 77 and 1451 nM for inhibition of radioligand binding to human NET, SERT and DAT respectively). Displays minimal affinity for a range of other neurotransmitter receptors and transporters (Ki > 1 μM). Antidepressant.
Product Specifications for Tomoxetine hydrochloride
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| water | 14.59 | 50 with gentle warming |
Preparing Stock Solutions for Tomoxetine hydrochloride
The following data is based on the product molecular weight 291.82.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 0.5 mM | 6.85 mL | 34.27 mL | 68.54 mL |
| 2.5 mM | 1.37 mL | 6.85 mL | 13.71 mL |
| 5 mM | 0.69 mL | 3.43 mL | 6.85 mL |
| 25 mM | 0.14 mL | 0.69 mL | 1.37 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 604 publications citing the usage of this product.
- Bymaster Atomoxetine increases extracellular levels of NE and DA in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002 PMID: 12431845
- Wong A new inhibitor of NE uptake devoid of affinity for receptors in rat brain. J.Pharmacol.Exp.Ther. 1982 PMID: 6123593
Product Documents for Tomoxetine hydrochloride
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Tomoxetine hydrochloride
For research use only
Citations for Tomoxetine hydrochloride
Customer Reviews for Tomoxetine hydrochloride (1)
Have you used Tomoxetine hydrochloride?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
-
Species: RatAssay Type: In VivoVerified Customer | Posted 07/24/2020diluted into saline solution to be injected into animals for observation of behavioral effects
There are no reviews that match your criteria.
