TPCA-1
Tocris Bioscience | Catalog # 2559

Key Product Details
Description
Product Description
TPCA-1 is a potent, selective inhibitor of IκB kinase (IKK) β (IC50 = 17.9 nM) that displays > 22-fold selectivity over IKKα and > 550-fold selectivity over other kinases and enzymes. Inhibits production of pro-inflammatory cytokines in vitro and in vivo and inhibits NF-κB nuclear localization. Reduces the severity and onset of collagen-induced arthritis; anti-inflammatory.
Product Specifications for TPCA-1
Molecular Weight
Formula
Storage
Purity
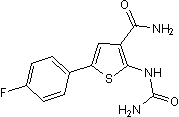
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 27.92 | 100 |
Preparing Stock Solutions for TPCA-1
The following data is based on the product molecular weight 279.29.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 3.58 mL | 17.90 mL | 35.81 mL |
| 5 mM | 0.72 mL | 3.58 mL | 7.16 mL |
| 10 mM | 0.36 mL | 1.79 mL | 3.58 mL |
| 50 mM | 0.07 mL | 0.36 mL | 0.72 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 73 publications citing the usage of this product.
- Birrell IK-B kinase-2 inhibitor blocks inflammation in human airway smooth muscle and a rat model of asthma. Am.J.Respir.Crit.Care Med. 2005 PMID: 16002568
- Birrell IκB kinase-2-independent and-dependent inflammation in airway disease models: relevance of IKK-2 inhibition to the clinic. Mol.Pharmacol. 2006 PMID: 16517756
- Podolin Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IκB kinase 2, TPCA-1 (2-[aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and J.Pharmacol.Exp.Ther. 2005 PMID: 15316093
Product Documents for TPCA-1
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for TPCA-1
For research use only
Related Research Areas
Citations for TPCA-1
Customer Reviews for TPCA-1
There are currently no reviews for this product. Be the first to review TPCA-1 and earn rewards!
Have you used TPCA-1?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
