Palmitoylethanolamide
Tocris Bioscience | Catalog # 0879

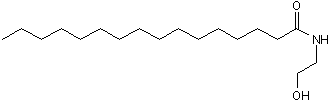
Key Product Details
Description
Alternative Names
Product Description
Palmitoylethanolamide is an endogenous lipid that acts as a selective GPR55 agonist (EC50 values are 4, 19 800 and > 30 000 nM at GPR55, CB2 and CB1 receptors respectively). Substrate for fatty acid amide hydrolase (FAAH) and PEA-preferring acid amidase (PAA) and exhibits antinociceptive and anticonvulsant in vivo. Directly activates PPARα (EC50 = 3 μM) producing robust anti-inflammatory actions.
Product Specifications for Palmitoylethanolamide
Molecular Weight
Formula
Storage
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 5.99 | 20 | |
| Ethanol | 7.49 | 25 |
Preparing Stock Solutions for Palmitoylethanolamide
The following data is based on the product molecular weight 299.50.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 0.25 mM | 13.36 mL | 66.78 mL | 133.56 mL |
| 1.25 mM | 2.67 mL | 13.36 mL | 26.71 mL |
| 2.5 mM | 1.34 mL | 6.68 mL | 13.36 mL |
| 12.5 mM | 0.27 mL | 1.34 mL | 2.67 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 18 publications citing the usage of this product.
- Ryberg The orphan receptor GPR55 is a novel cannabinoid receptor. Br.J.Pharmacol. 2007 PMID: 17876302
- Re Palmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: potential use in companion animals. Vet.J. 2005 PMID: 16324856
- Lambert The palmitoylethanolamide family: a new class of anti-inflammatory agents? Curr.Med.Chem. 2002 PMID: 11945130
- Lo Verme The search for the palmitoylethanolamide receptor. Life Sci. 2005 PMID: 15963531
- Lambert Anticonvulsant activity of N-palmitoylethanolamide, a putative endocannabinoid, in mice. Epilepsia 2001 PMID: 11442148
Product Documents for Palmitoylethanolamide
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Palmitoylethanolamide
For research use only
Citations for Palmitoylethanolamide
Customer Reviews for Palmitoylethanolamide
There are currently no reviews for this product. Be the first to review Palmitoylethanolamide and earn rewards!
Have you used Palmitoylethanolamide?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
