TLR9 Antibody (2A4C6.2E5) - BSA Free
Novus Biologicals | Catalog # NBP2-31150

Key Product Details
Species Reactivity
Mouse
Applications
Immunohistochemistry, Immunohistochemistry-Paraffin, Western Blot, ICC/IF (Negative)
Label
Unconjugated
Antibody Source
Monoclonal Rat IgG2a Kappa Clone # 2A4C6.2E5
Format
BSA Free
Loading...
Product Specifications
Immunogen
Partial recombinant protein made to an internal portion of mouse TLR9 (between amino acids 500-800) [UniProt Q9EQU3]
Localization
Endoplasmic reticulum membrane, Endosome, Lysosome, Cytoplasmic vesicle/phagosome.
Clonality
Monoclonal
Host
Rat
Isotype
IgG2a Kappa
Description
Novus Biologicals Rat TLR9 Antibody (2A4C6.2E5) - BSA Free (NBP2-31150) is a monoclonal antibody validated for use in IHC and WB. All Novus Biologicals antibodies are covered by our 100% guarantee.
Scientific Data Images for TLR9 Antibody (2A4C6.2E5) - BSA Free
Western Blot: TLR9 Antibody (2A4C6.2E5)BSA Free [NBP2-31150]
Western Blot: TLR9 Antibody (2A4C6.2E5) [NBP2-31150] - Mouse Liver (lane 1), Mouse Kidney (lane 2) and Mouse Spleen (lane 3) were separated by SDS-PAGE and the protein transferred to PVDF. The membrane was then probed with anti-TLR9 at 2ug/mL and detected with an anti-rat HRP secondary antibody using chemiluminescence.Immunohistochemistry-Paraffin: TLR9 Antibody (2A4C6.2E5) - BSA Free [NBP2-31150]
Immunohistochemistry-Paraffin: TLR9 Antibody (2A4C6.2E5) [NBP2-31150] - Analysis of TLR9 protein in normal mouse epididymis section using TLR9 antibody (clone 2E5) at a concentration of 5 ug/mL. This antibody developed strong cytoplasmic staining in the pseudostratified columnar epithelial cells as well as the blood vessels of epididymis. The basal cells, the smooth muscle cells and the mature sperm cells were largely found negative for TLR9 staining.Western Blot: TLR9 Antibody (2A4C6.2E5)BSA Free [NBP2-31150]
Western Blot: TLR9 Antibody (2A4C6.2E5) [NBP2-31150] - TLR9 Antibody (2E5) [NBP2-31150] - TLR9 Antibody (2E5) [NBP2-31150] - WB analysis of partial recombinant TLR9 protein (expected molecular weight ~21kDa) with TLR9 antibody (clone 2A4C6.2E5) at a concentration of 2 ug/mL.Applications for TLR9 Antibody (2A4C6.2E5) - BSA Free
Application
Recommended Usage
Immunohistochemistry
2 ug/mL
Immunohistochemistry-Paraffin
2-5 ug/mL
Western Blot
2 ug/mL
Formulation, Preparation, and Storage
Purification
Protein G purified
Formulation
PBS
Format
BSA Free
Preservative
0.05% Sodium Azide
Concentration
1.0 mg/ml
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Store at 4C short term. Aliquot and store at -20C long term. Avoid freeze-thaw cycles.
Background: TLR9
Long Name
Toll-like Receptor 9
Alternate Names
CD289
Entrez Gene IDs
81897 (Mouse)
Gene Symbol
TLR9
UniProt
Additional TLR9 Products
Product Documents for TLR9 Antibody (2A4C6.2E5) - BSA Free
Product Specific Notices for TLR9 Antibody (2A4C6.2E5) - BSA Free
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Primary Antibodies are guaranteed for 1 year from date of receipt.
Customer Reviews for TLR9 Antibody (2A4C6.2E5) - BSA Free
There are currently no reviews for this product. Be the first to review TLR9 Antibody (2A4C6.2E5) - BSA Free and earn rewards!
Have you used TLR9 Antibody (2A4C6.2E5) - BSA Free?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Protocols
View specific protocols for TLR9 Antibody (2A4C6.2E5) - BSA Free (NBP2-31150):
TLR9 Antibody (2A4C6.2E5):
1. Deparaffinize the tissue sections by immersing the slides in Xylene with two changes for 10 min each. Sections should not get dried at any stage from this point.
2. Rehydrate the tissue sections by immersing the slides in decreasing grades of ethanol as follows:
a. Immerse in 100% ethanol with 2 changes for 5 minutes each
b. Immerse in 95% ethanol with 2 changes for 5 minutes each
c. Immerse in 90% ethanol for 5 minutes
d. Immerse in 70% ethanol for 5 minutes
e. Immerse in 50% ethanol for 5 minutes
f. Immerse in distilled water for 5 minutes
3. Antigen Retrieval (Microwave Method):
a. Immerse the slides in a microwave compatible tray containing 10 mM Sodium Citrate buffer (pH 6.0) with 0.05% Tween 20.
b. Boil the slides and maintain the sub-boiling temperature for 5 minutes in the microwave. Thereafter, take out the tray very carefully and cool it at room temperature (RT) for about 30 minutes.
c. Wash the slides 3 times, 3 minutes each by immersing them in TBST (Tris Buffered Saline having 0.05% Tween 20).
4. Quenching of Endogenous Peroxidase:
a. Incubate the slides in 3% hydrogen peroxide prepared in methanol for 15 minutes (at RT, in dark conditions).
b. Wash the slides in TBST 3 times, 3 minutes each.
5. Protein Blocking:
a. Incubate the sections with background sniper solution at RT for 15 minutes (Biocare Medicals, USA).
b. Wash the sections 3 times, 3 min each by immersing the slides in TBST.
6. Primary Antibody:
a. Dilute the primary antibody at 5ug/ml concentration using PBS as a diluent.
b. Incubate the sections with diluted primary antibody for 90 minutes at RT in a humidified chamber.
c. Thereafter, wash the slides 4 times, 5 minutes each with TBST.
7. Probe (Secondary Reagent):
a. Incubate with MACH 1 Mouse probe for 15 minutes at RT.
b. Incubate for 30 min at room temperature with HRP-Polymer (Biocare Medical, USA).
c. Wash the slides with TBST 4 times, 5 minutes each
8. Chromogen:
a. Mix 32ul of DAB Chromogen with 1 ml of DAB substrate buffer (Biocare Medical, USA).
b. Apply 200ul DAB mixture/section and incubate at RT in dark conditions (few seconds - 5 minutes).
c. As soon as an appropriate color develops, rinse the slides with deionized water (2-3 brief rinses).
9. Counter stain:
a. Counter stain with Hematoxylin for 30 seconds (Vector Labs, USA).
b. Wash in deionized water for 1-2 minutes to clear the extra stain.
c. Incubate the slides in bluing solution or Scott's water twice for 2 minutes each time.
10. Dehydrate the sections in increasing grades of alcohols:
a. 50% alcohol for 1 minute
b. 70% for 1 minute
c. 90% for 1 minute
d. 95% for 1 minute
e. 100% for 1 minute
f. Xylene with 2 changes for 2 minutes each
11. Mount with DPX mount and cover-slip glass (Fisher Scientific, USA), carefully not allowing any air bubbles to enter.
NOTE:- This protocol is provided as a reference tool only. Depending upon the type of tissues /tissue processing and reagents employed, the end user will need to optimize the final conditions for achieving an expected staining.
1. Deparaffinize the tissue sections by immersing the slides in Xylene with two changes for 10 min each. Sections should not get dried at any stage from this point.
2. Rehydrate the tissue sections by immersing the slides in decreasing grades of ethanol as follows:
a. Immerse in 100% ethanol with 2 changes for 5 minutes each
b. Immerse in 95% ethanol with 2 changes for 5 minutes each
c. Immerse in 90% ethanol for 5 minutes
d. Immerse in 70% ethanol for 5 minutes
e. Immerse in 50% ethanol for 5 minutes
f. Immerse in distilled water for 5 minutes
3. Antigen Retrieval (Microwave Method):
a. Immerse the slides in a microwave compatible tray containing 10 mM Sodium Citrate buffer (pH 6.0) with 0.05% Tween 20.
b. Boil the slides and maintain the sub-boiling temperature for 5 minutes in the microwave. Thereafter, take out the tray very carefully and cool it at room temperature (RT) for about 30 minutes.
c. Wash the slides 3 times, 3 minutes each by immersing them in TBST (Tris Buffered Saline having 0.05% Tween 20).
4. Quenching of Endogenous Peroxidase:
a. Incubate the slides in 3% hydrogen peroxide prepared in methanol for 15 minutes (at RT, in dark conditions).
b. Wash the slides in TBST 3 times, 3 minutes each.
5. Protein Blocking:
a. Incubate the sections with background sniper solution at RT for 15 minutes (Biocare Medicals, USA).
b. Wash the sections 3 times, 3 min each by immersing the slides in TBST.
6. Primary Antibody:
a. Dilute the primary antibody at 5ug/ml concentration using PBS as a diluent.
b. Incubate the sections with diluted primary antibody for 90 minutes at RT in a humidified chamber.
c. Thereafter, wash the slides 4 times, 5 minutes each with TBST.
7. Probe (Secondary Reagent):
a. Incubate with MACH 1 Mouse probe for 15 minutes at RT.
b. Incubate for 30 min at room temperature with HRP-Polymer (Biocare Medical, USA).
c. Wash the slides with TBST 4 times, 5 minutes each
8. Chromogen:
a. Mix 32ul of DAB Chromogen with 1 ml of DAB substrate buffer (Biocare Medical, USA).
b. Apply 200ul DAB mixture/section and incubate at RT in dark conditions (few seconds - 5 minutes).
c. As soon as an appropriate color develops, rinse the slides with deionized water (2-3 brief rinses).
9. Counter stain:
a. Counter stain with Hematoxylin for 30 seconds (Vector Labs, USA).
b. Wash in deionized water for 1-2 minutes to clear the extra stain.
c. Incubate the slides in bluing solution or Scott's water twice for 2 minutes each time.
10. Dehydrate the sections in increasing grades of alcohols:
a. 50% alcohol for 1 minute
b. 70% for 1 minute
c. 90% for 1 minute
d. 95% for 1 minute
e. 100% for 1 minute
f. Xylene with 2 changes for 2 minutes each
11. Mount with DPX mount and cover-slip glass (Fisher Scientific, USA), carefully not allowing any air bubbles to enter.
NOTE:- This protocol is provided as a reference tool only. Depending upon the type of tissues /tissue processing and reagents employed, the end user will need to optimize the final conditions for achieving an expected staining.
TLR9 Antibody (2A4C6.2E5):
Western Blot Protocol
1. Perform SDS-PAGE on samples to be analyzed, loading 25 ug of total protein per lane.
2. Transfer proteins to membrane according to the instructions provided by the manufacturer of the membrane and transfer apparatus.
3. Stain according to standard Ponceau S procedure (or similar product) to assess transfer success, and mark molecular weight standards where appropriate.
4. Rinse the blot.
5. Block the membrane using standard blocking buffer for at least 1 hour.
6. Wash the membrane in wash buffer three times for 10 minutes each.
7. Dilute anti-TLR9 primary antibody in blocking buffer and incubate 1 hour at room temperature.
8. Wash the membrane in wash buffer three times for 10 minutes each.
9. Apply the diluted HRP conjugated secondary antibody in blocking buffer (as per manufacturers instructions) and incubate 1 hour at room temperature.
10. Wash the blot in wash buffer three times for 10 minutes each (this step can be repeated as required to reduce background).
11. Apply the detection reagent of choice in accordance with the manufacturers instructions.
Note: Tween-20 can be added to the blocking or antibody dilution buffer at a final concentration of 0.05-0.2%.
Western Blot Protocol
1. Perform SDS-PAGE on samples to be analyzed, loading 25 ug of total protein per lane.
2. Transfer proteins to membrane according to the instructions provided by the manufacturer of the membrane and transfer apparatus.
3. Stain according to standard Ponceau S procedure (or similar product) to assess transfer success, and mark molecular weight standards where appropriate.
4. Rinse the blot.
5. Block the membrane using standard blocking buffer for at least 1 hour.
6. Wash the membrane in wash buffer three times for 10 minutes each.
7. Dilute anti-TLR9 primary antibody in blocking buffer and incubate 1 hour at room temperature.
8. Wash the membrane in wash buffer three times for 10 minutes each.
9. Apply the diluted HRP conjugated secondary antibody in blocking buffer (as per manufacturers instructions) and incubate 1 hour at room temperature.
10. Wash the blot in wash buffer three times for 10 minutes each (this step can be repeated as required to reduce background).
11. Apply the detection reagent of choice in accordance with the manufacturers instructions.
Note: Tween-20 can be added to the blocking or antibody dilution buffer at a final concentration of 0.05-0.2%.
Find general support by application which include: protocols, troubleshooting, illustrated assays, videos and webinars.
- Antigen Retrieval Protocol (PIER)
- Antigen Retrieval for Frozen Sections Protocol
- Appropriate Fixation of IHC/ICC Samples
- Cellular Response to Hypoxia Protocols
- Chromogenic IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Chromogenic Immunohistochemistry Staining of Frozen Tissue
- Detection & Visualization of Antibody Binding
- Fluorescent IHC Staining of Frozen Tissue Protocol
- Graphic Protocol for Heat-induced Epitope Retrieval
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Graphic Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- IHC Sample Preparation (Frozen sections vs Paraffin)
- Immunofluorescent IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Immunohistochemistry (IHC) and Immunocytochemistry (ICC) Protocols
- Immunohistochemistry Frozen Troubleshooting
- Immunohistochemistry Paraffin Troubleshooting
- Preparing Samples for IHC/ICC Experiments
- Preventing Non-Specific Staining (Non-Specific Binding)
- Primary Antibody Selection & Optimization
- Protocol for Heat-Induced Epitope Retrieval (HIER)
- Protocol for Making a 4% Formaldehyde Solution in PBS
- Protocol for VisUCyte™ HRP Polymer Detection Reagent
- Protocol for the Preparation & Fixation of Cells on Coverslips
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections - Graphic
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections - Graphic
- Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- R&D Systems Quality Control Western Blot Protocol
- TUNEL and Active Caspase-3 Detection by IHC/ICC Protocol
- The Importance of IHC/ICC Controls
- Troubleshooting Guide: Immunohistochemistry
- Troubleshooting Guide: Western Blot Figures
- Western Blot Conditions
- Western Blot Protocol
- Western Blot Protocol for Cell Lysates
- Western Blot Troubleshooting
- Western Blot Troubleshooting Guide
- View all Protocols, Troubleshooting, Illustrated assays and Webinars
Loading...
Associated Pathways
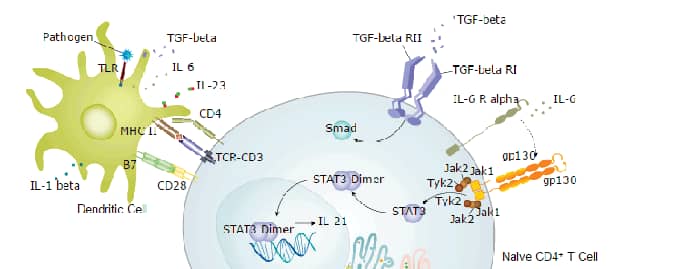
 IL-21 Signaling Pathways and their Primary Biological Effects in Different Immune Cell Types
IL-21 Signaling Pathways and their Primary Biological Effects in Different Immune Cell Types
 Inflammasome Activation Pathways
Inflammasome Activation Pathways
 NOD-like Receptor Signaling Pathways
NOD-like Receptor Signaling Pathways
 Pathogen or Damage-activated C-Type Lectin Receptor Signaling Pathways
Pathogen or Damage-activated C-Type Lectin Receptor Signaling Pathways


![Immunohistochemistry-Paraffin: TLR9 Antibody (2A4C6.2E5) - BSA Free [NBP2-31150] Immunohistochemistry-Paraffin: TLR9 Antibody (2A4C6.2E5) - BSA Free [NBP2-31150]](https://resources.rndsystems.com/images/products/TLR9-Antibody-2E5-Immunohistochemistry-Paraffin-NBP2-31150-img0006.jpg)
![Western Blot: TLR9 Antibody (2A4C6.2E5)BSA Free [NBP2-31150] Western Blot: TLR9 Antibody (2A4C6.2E5)BSA Free [NBP2-31150]](https://resources.rndsystems.com/images/products/TLR9-Antibody-2E5-Western-Blot-NBP2-31150-img0004.jpg)
![Western Blot: TLR9 Antibody (2A4C6.2E5)BSA Free [NBP2-31150] Western Blot: TLR9 Antibody (2A4C6.2E5)BSA Free [NBP2-31150]](https://resources.rndsystems.com/images/products/TLR9-Antibody-2E5-Western-Blot-NBP2-31150-img0007.jpg)