Epitope tags such as V5, c-myc, and HA are biological structures, usually proteins, that are genetically engineered onto either the N or C terminus of a recombinant protein. Tags function as universal epitopes, easily detected by commercially available antibodies, and generally do not compromise the native structure or function of the protein.

What Are Epitope Tags Used For?
Tags and anti-tag antibodies are useful for many different immunoassays such as western blotting, immunoprecipitation, immunohistochemistry, immunocytochemistry, and flow cytometry. If your epitope tag ever loses antigenicity following deparaffinization and rehydration of formalin-fixed tissues in immunohistochemistry, try following our antigen retrieval protocol. Epitope tags are the most economical way to detect newly discovered proteins for which specific antibodies are not yet available or for targets that are poorly immunogenic 1. Additionally, epitope tags are also useful for affinity purification of target proteins from cell lysates 2, enhancing the solubility of target proteins 3 protecting against intracellular protease cleavage (e.g. GST) 4, and adding enzymatic function to a target protein 5.

Western Blot
V5 Epitope Tag Antibody [NB600-381] - Detection of 200, 100, or 50 ng of E. coli whole cell lysate expressing a multi-tag fusion protein.
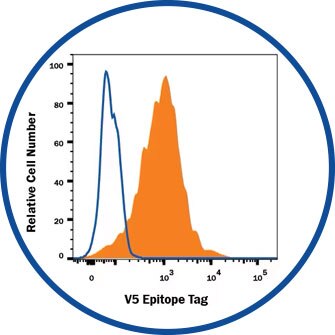
Flow Cytometry
V5 Epitope Tag Antibody [MAB8926] - HEK293 human embryonic kidney cell line transfected with V5-tagged proteins.

Immunocytochemistry/Immunofluorescence
HA Tag Antibody [NB600-363] - Detection of HA-tagged Tubulin in CHO cells transfected with mutant beta-tubulin cDNA encoding an HA epitope tag.

Immunohistochemistry-Paraffin
c-Myc Antibody (9E10) [NB600-302] - c-Myc was detected in immersion fixed paraffin-embedded sections of human breast cancer using anti-human mouse monoclonal antibody.

Affinity Purification
HA, His, DYKDDDDK, and V5 are commonly used epitope tags for affinity purification.
Choosing Your Epitope Tag
No single epitope tag is useful for every application. When choosing a tag, consider how the size and charge of the tag will ultimately affect the protein’s function, solubility and downstream analysis. Multiple tags can be added to a single protein to get enhanced functionality, however, this increases the risk of losing native function, compromising the original structure, or altering protein trafficking 6,7,8.
Peptide Tags
| Epitope Tags | Length (Sequence) (kDa) | Alternative Names | Source | Notes |
|---|---|---|---|---|
| HA | YPYDVPDYA | Hemagglutinin | Amino acids 98-106 of Human influenza hemagglutin which is a surface glycoprotein required for the infectivity of the human virus | Strong immunoreactive epitope and mild elution conditions makes it a popular epitope tag to purify tagged proteins 10; useful in mammalian expression systems 9; not suitable for detection or purification of proteins from apoptotic cells since it is cleaved by Caspase-3 and / or Caspase-7 after its sequence DVPD, causing it to lose its immunoreactivity 11 |
| HIS | H-H-H-H-H-H | 6 His 6X-His HHHHHH tag polyHistidine hexa-histidine tag | Synthetic | Most common purification tag; Purification via Nickel, Cobalt, Copper, or Zinc column; Regenerable affinity matrix 9 |
| FLAGTM | DYKDDDDK | Ty 1 Tag DDK tag DDDDK tag DYKDDDDK tag xxxDDDDK epitope tag Enterokinase Cleavage Site tag ECS tag Ty 1 | Synthetic | Hydrophilic epitope tag introduced to purify fusion proteins 10; Contains internal enterokinase cleavage site |
| AU1 | DTYRYI | Major capsid protein of bovine papillomavirus-1 (BPV-1) | Not recommended for affinity purification as protein properties may get altered during low pH elution step 9 | |
| AU5 | TDFYLK | Major capsid protein of bovine paillomavirus-1 (BPV-1) | Not recommended for affinity purification as protein properties may get altered during low pH elution step 9 | |
| Myc | EQKLISEEDL | Amino acid residues 410-419 of human c-Myc | Not recommended for affinity purification as protein properties may get altered during low pH elution step 9 | |
| Glu-Glu | EYMPME | Amino acid residues 314-319 of middle T antigen of mouse polyomavirus | Not recommended for affinity purification as protein properties may get altered during low pH or 30°C elution step 9 | |
| OLLAS | SGFANELGPRLMGK | OLLA-2 E. coli OmpF linker and mouse Langerin fusion sequence | 14 amino acid sequence residing in the junction between E. coli OmpF protein (OFL/OmpF linker) and mLangerin extracellular domain | The OLLAS epitope tag is detectable at approximately 100-fold lower levels than similar commercially available tags |
| T7 | MASMTGGQQMG | 11 amino acid N-terminus of Bacteriophage T7 gene 10 | May help increase expression; Not recommended for affinity purification as protein properties may get altered during low pH elution step 9 | |
| V5 | GKPIPNPLLGLDST | Parainfluenza virus 5 V/P tag Paramyxovirus SV5 tag | Amino acid residues 95 to 108 of RNA polymerase alpha subunit of simian virus 5 | Some cross reactivity may occur when using mammalian expression systems; Recommended for affinity purification in combination with His-tag 9 |
| VSV-G | YTDIEMNRLGK | Vesicular stomatitis virus glycoprotein tag | 11 amino acid C-terminus of the vesicular stomatitis viral glycoprotein | Not recommended for affinity purification as protein properties may get altered during low pH elution step 9 |
| E-Tag | GAPVPYPDPLEPR | Bone hormone osteocalcin produced by osteoblasts | ||
| S-Tag | KETAAAKFERQHMDS | S 7 Epitope tag | 15 amino acid sequence at N-terminus of RNase A | Not recommended for affinity purification as protein properties may get altered during low pH elution step 9 |
| Avi | CGLNDIFEAQKIEWHE | Synthetic | Allows for either the in vivo or in vitro enzymatic biotinylation by the biotin ligase BirA from E. coli; May lead to decreased solubility 12 | |
| HSV | SQPELAPEDPED | Herpes simplex virus tag | C-terminal placement only; Not recommended for affinity purification as protein properties may get altered during low pH elution step 9 | |
| KT3 | KPPTPPPEPET | Simian virus 40 (SV40) large T-antigen | Not recommended for affinity purification as protein properties may get altered during low pH elution step 9 | |
| TK15 | (R/K)TV(I/L)HGESNSA(I/L)(I/L)(I/L)GPR | Xenopus Orc1p | No cross-reactivity with mammalian or bacterial proteins has been detected; Used for immunoaffinity purification 16 | |
| Strep-tag II | WSHPQFEK or AWAHPQPGG | Synthetic | Regenerable affinity matrix; compatible with purifications requiring anaerobic conditions 9 | |
| Beta-Galactosidase | 116 kDa | Enzyme coded by lac z gene in the lac operon of E. coli | Metalloenzyme that splits lactose into glucose and galactose; Allows for enzymatic protein quantification assays; May protect from proteolytic activity; May decrease solubility; Tag may form tetramers in solution 9 |
Fusion Proteins
| Epitope Tags | Length (Sequence) (kDa) | Alternative Names | Source | Notes |
|---|---|---|---|---|
| Maltose Binding Protein | 42.5 kDa | MBP | Maltose/maltodextrin system of E. coli | Amylose purification column compatible with non-ionizing detergents and high salt but not reducing agents; Large size; N-terminal tagging may reduce translation efficiency; 9; Enhanced protein production 13 |
| Calmodulin Binding Protein (CBP) | 4 kDa | Cytoplasm of all Eukaryotic cells | No cross-reaction with any endogenous E.coli proteins; High yield affinity Calmodulin column purification; Not useful for purification from eukaryotic cells; N-terminus tagging may reduce translation efficiency 9 | |
| Green Fluorescent Protein (GFP) | 27 kDa | Jellyfish Aequorea victoria | Major excitation peak at a wavelength of 395 nm and a minor one at 475 nm; emission peak is at 509 nm; Detectable in living organisms without antibody; Can sometimes be non-specifically directed to nucleus; Useful to monitor protein-protein interactions by FRET; Very large; Dimerization may occur 9; Useful in protein-folding assays 5 | |
| Glutathione S Transferase (GST) | 27 kDa | Eukaryotes and prokaryotes | Affinity purification via relatively reusable glutathione conjugated columns; purification under native conditions only; Highly antigenic; may cause insolubility; may dimerize 9 | |
| mCherry | 28 kDa | DsRED Red Fluorescent Protein RFP | Disc corals of Discosoma species | Useful to monitor protein-protein interactions by FRET; Excitation maximum at 587 nm and an emission maximum at 610 nm |
| Fc-Fusion Proteins | Fc chimeric fusion proteins Fc-Ig's Fc-tag protein Ig-based Chimeric fusion protein | Fc domain of IgG Antibody | The Fc domain can improve the solubility and stability of the tagged protein both in vitro and in vivo; Easy cost-effective purification by protein-G/A affinity chromatography 14 | |
| Thioredoxin | 12 kDa | Trx TRDX | Found in all organisms; Encoded by TXN and TXN2 genes in humans | Can enhance the solubility of tagged protein 15 |
Cleavage Sites
Tags are removable by proteolytic enzymes that cleave the tag from the protein of interest, and generally the cleavage site needs to be engineered between the tag and the protein. Consider utilizing these commonly used proteases in your experiments: (Note: // denotes cleavage site)
| Protease | Recognition Site |
|---|---|
| EV Protease | Glu-Asn-Leu-Tyr-Phe-Gln-Gly//Ser |
| Thrombin | Leu-Val-Pro-Arg//Gly-Ser |
| Factor Xa | Ile-(Glu/Asp)-Gly-Arg// Occasionally Gly-Arg// |
| Enteropeptidase/Enterokinase | Asp-Asp-Asp-Asp-Lys// |
GST Tag Removal
GST tag Removal using PreScission Protease
GST tags may be removed using PreScission Proteases. Recombinant PreScission Protease from Bio-Techne is a fusion protein consisting of glutathione S-transferase (GST) and human rhinovirus (HRV) type 14 3C protease. The protease specifically recognizes a subset of sequences which include the core amino acid sequence Leu-Phe-Gln/Gly-Pro cleaving between the Gln and Gly residues. The amount of PreScission Protease, temperature, and incubation time required for complete digestion of a given GST fusion partner may differ depending on the fusion partner. One unit of PreScission Protease is defined as the amount of enzyme needed to cleave 100 µg of fusion protein in 16 hours to 90% completion at 5°C in a buffer containing 50 mM Tris-HCl, pH 7.0, 150 mM NaCl, 1 mM EDTA, and 1 mM DTT.
References
- Brizzard, B (2018) Mini-Review: Epitope Tagging. BioTechniques, 44 693. https://doi.org/10.2144/000112841
- Kimple ME, Brill AL, Pasker RL. Overview of affinity tags for protein purification. Curr Protoc Protein Sci. 2013 73 Unit 9.9. doi:10.1002/0471140864.ps0909s73
- Walls D. & Loughran S.T. (2011) Tagging Recombinant Proteins to Enhance Solubility and Aid Purification. In: Walls D., Loughran S. (eds) Protein Chromatography. Methods in Molecular Biology (Methods and Protocols), vol 681. Humana Press
- Terpe, K. Appl Microbiol Biotechnol (2003) 60: 523. https://doi.org/10.1007/s00253-002-1158-6
- Waldo GS, Standish BM, Berendzen J, Terwilliger TC. (1999) Rapid protein-folding assay using green fluorescent protein. Nat Biotechnol. Jul;17(7):691-5.
- Sabourin, M., Tuzon, C. T., Fisher, T. S. and Zakian, V. A. (2007), A flexible protein linker improves the function of epitope‐tagged proteins in Saccharomyces cerevisiae. Yeast, 24: 39-45. doi:10.1002/yea.1431
- Zordan RE, Beliveau BJ, Trow JA, Craig NL, Cormack BP. Avoiding the ends: internal epitope tagging of proteins using transposon Tn7. Genetics. 2015;200(1):47-58.
- Maue, R. A. (2007), Understanding ion channel biology using epitope tags: Progress, pitfalls, and promise. J. Cell. Physiol., 213: 618-625. doi:10.1002/jcp.21259
- Zhao X, Li G, Liang S. Several affinity tags commonly used in chromatographic purification. J Anal Methods Chem. 2013;2013:581093.
- Schembri L, Dalibart R, Tomasello F, Legembre P, Ichas F, De Giorgi F. (2007) The HA tag is cleaved and loses immunoreactivity during apoptosis. Nat Methods. Feb;4(2):107-8.
- Mukherjee S, Ura M, Hoey RJ, Kossiakoff AA. A New Versatile Immobilization Tag Based on the Ultra High Affinity and Reversibility of the Calmodulin-Calmodulin Binding Peptide Interaction. J Mol Biol. 2015;427(16):2707-25.
- Reuten R, Nikodemus D, Oliveira MB, et al. Maltose-Binding Protein (MBP), a Secretion-Enhancing Tag for Mammalian Protein Expression Systems. PLoS One. 2016;11(3):e0152386. Published 2016 Mar 30. doi:10.1371/journal.pone.0152386
- Carter PJ. (2011) Exp Cell Res. Introduction to current and future protein therapeutics: a protein engineering perspective. May 15;317(9):1261-9.
- Yasukawa T, Kanei-Ishii C, Maekawa T, Fujimoto J, Yamamoto T, Ishii S. (1995) Increase of solubility of foreign proteins in Escherichia coli by coproduction of the bacterial thioredoxin. J Biol Chem. Oct 27;270(43):25328-31.
- Tugal T, Zou-Yang XH, Gavin K, Pappin D, et al. (1998) The Orc4p and Orc5p subunits of the Xenopus and human origin recognition complex are related to Orc1p and Cdc6p. J Biol Chem. Dec 4;273(49):32421-9.
*FLAG is a trademark of Sigma Aldrich.
