![Immunohistochemistry: Mas Antibody - BSA Free [NLS1531] Immunohistochemistry: Mas Antibody - BSA Free [NLS1531]](https://resources.rndsystems.com/images/products/Mas-Antibody-Immunohistochemistry-NLS1531-img0001.jpg)
Key Product Details
Species Reactivity
Validated:
Human, Mouse, Rat
Cited:
Human
Applications
Validated:
Immunohistochemistry, Immunohistochemistry-Paraffin, Immunohistochemistry-Frozen, Flow Cytometry, Immunocytochemistry/ Immunofluorescence
Cited:
Flow Cytometry, Immunocytochemistry/ Immunofluorescence
Label
Unconjugated
Antibody Source
Polyclonal Rabbit IgG
Format
BSA Free
Loading...
Product Specifications
Immunogen
KLH conjugated synthetic peptide made to the C-terminus of human MAS1. [UniProt# P04201]
Localization
Cell membrane
Clonality
Polyclonal
Host
Rabbit
Isotype
IgG
Description
Novus Biologicals Rabbit Mas Antibody - BSA Free (NLS1531) is a polyclonal antibody validated for use in IHC, Flow and ICC/IF. Anti-Mas Antibody: Cited in 4 publications. All Novus Biologicals antibodies are covered by our 100% guarantee.
Scientific Data Images for Mas Antibody - BSA Free
Immunohistochemistry: Mas Antibody - BSA Free [NLS1531]
Immunohistochemistry: Mas Antibody [NLS1531] - Analysis of MAS1 in mouse kidney using DAB with hematoxylin counterstain.Immunohistochemistry-Paraffin: Mas Antibody - BSA Free [NLS1531]
Mas-Antibody-Immunohistochemistry-Paraffin-NLS1531-img0003.jpgImmunohistochemistry-Paraffin: Mas Antibody - BSA Free [NLS1531]
Immunohistochemistry-Paraffin: Mas Antibody [NLS1531] - Analaysis of Brain, Neurons and Glia using NLS1531 at 15 ug/ ml.Immunohistochemistry: Mas Antibody - BSA Free [NLS1531] -
(a) Representative immunolocalisation images of AT1R, AT2R, MasR, and ACE2 in adult WKY rats and aged WKY rats. Mean data for aortic expression of the (b) AT1R, (c) AT2R, (d) MasR, and (e) ACE2 expressed as relative fluorescent units in adult (n = 5) and aged (n = 4) WKY rats. ***P < 0.001 versus adult WKY rats.Immunocytochemistry/ Immunofluorescence: Mas Antibody - BSA Free [NLS1531] -
Immunocytochemistry/ Immunofluorescence: Mas Antibody - BSA Free [NLS1531] - (a) Representative immunolocalisation images of AT1R, AT2R, MasR, & ACE2 in adult WKY rats & aged WKY rats. Mean data for aortic expression of the (b) AT1R, (c) AT2R, (d) MasR, & (e) ACE2 expressed as relative fluorescent units in adult (n = 5) & aged (n = 4) WKY rats. ***P < 0.001 versus adult WKY rats. Image collected & cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/22187625), licensed under a CC-BY license. Not internally tested by Novus Biologicals.Immunocytochemistry/ Immunofluorescence: Mas Antibody - BSA Free [NLS1531] -
Immunocytochemistry/ Immunofluorescence: Mas Antibody - BSA Free [NLS1531] - Immunofluorescence studies in HEK293T cells overexpressing c-myc tagged MasR using MasR antibodies.Images of fluorescence signal corresponding to secondary cyanine (Cy3)-conjugated antibody (A), nuclear Hoechst 33258 staining (B), merged images (C) & bright field (D) are shown. In cells transfected with the pcDNA 3.1/c-myc-MasR construct, antibodies NLS-1531 & sc-54682 generated a similar staining pattern to that generated with the anti c-myc antibody. No staining was observed with any of these antibodies in cells transfected with the empty vector pcDNA 3.1. Antibody sc-135063 was capable of staining the plasma membrane of cells overexpressing the MasR but also generated widespread signals in cells transfected with the empty vector. For antibody AAR-013 there was no staining associated with the cell membrane. Intense immunocytochemical staining of identical distribution & intensity was revealed in cells transfected with the empty vector & those transfected with the c-myc-MasR construct. Images are representative of 3 independent experiments. Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0183278), licensed under a CC-BY license. Not internally tested by Novus Biologicals.Immunohistochemistry: Mas Antibody - BSA Free [NLS1531] -
Immunohistochemistry: Mas Antibody - BSA Free [NLS1531] - Immunohistochemistry in heart & kidney from wild-type (WT) & MasR-KO mice.Negative controls performed by omitting the primary antibody demonstrated minimal background immunostaining in heart (A) & kidney (B) sections. (A) In heart sections, NLS-1531 antibody stained predominantly the cytoplasm of cardiomyocytes with similar intensity in WT & MasR-KO hearts. The AAR-013 antibody stained the cardiomyocytes nucleus & weaker staining was observed in their cytoplasm. The same pattern was found in heart sections of MasR-KO mice. (B) In kidney sections, for NLS-1531 antibody, staining was mostly restricted to cytoplasm of numerous tubules cells with similar intensity in WT & MasR-KO kidneys. Antibody AAR-013 stained most intensely tubules cells nucleus & weaker staining was observed in their cytoplasm. Glomeruli were also stained positively. The same pattern was found in kidney sections of MasR-KO. Preincubation of the AAR-013 antibody with the blocking peptide provided by the vendors eliminated the immunohistochemical nuclear staining both in WT & MasR-KO mice kidney sections. Images are representative of n = 3. Bar, 50 μm. Image collected & cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0183278), licensed under a CC-BY license. Not internally tested by Novus Biologicals.Applications for Mas Antibody - BSA Free
Application
Recommended Usage
Flow Cytometry
reported in scientific literature (PMID 26386115)
Immunocytochemistry/ Immunofluorescence
1:10-1:500. Use reported in scientific literature (PMID 22187625)
Immunohistochemistry
1:100-1:500
Immunohistochemistry-Frozen
1:100-1:500
Immunohistochemistry-Paraffin
1:100-1:500. Use reported in scientific literature (PMID 22187625)
Application Notes
Prior to immunostaining paraffin tissues, antigen retrieval with sodium citrate buffer (pH 6.0) is recommended.
Reviewed Applications
Read 1 review rated 5 using NLS1531 in the following applications:
Flow Cytometry Panel Builder
Bio-Techne Knows Flow Cytometry
Save time and reduce costly mistakes by quickly finding compatible reagents using the Panel Builder Tool.
Advanced Features
- Spectra Viewer - Custom analysis of spectra from multiple fluorochromes
- Spillover Popups - Visualize the spectra of individual fluorochromes
- Antigen Density Selector - Match fluorochrome brightness with antigen density
Formulation, Preparation, and Storage
Purification
Immunogen affinity purified
Formulation
PBS
Format
BSA Free
Preservative
0.01% Sodium Azide
Concentration
1 mg/ml
Shipping
The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below.
Stability & Storage
Store at 4C short term. Aliquot and store at -20C long term. Avoid freeze-thaw cycles.
Background: Mas
Additional Mas Products
Product Documents for Mas Antibody - BSA Free
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Mas Antibody - BSA Free
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Primary Antibodies are guaranteed for 1 year from date of receipt.
Related Research Areas
Citations for Mas Antibody - BSA Free
Customer Reviews for Mas Antibody - BSA Free (1)
5 out of 5
1 Customer Rating
Have you used Mas Antibody - BSA Free?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
Showing
1
-
1 of
1 review
Showing All
Filter By:
-
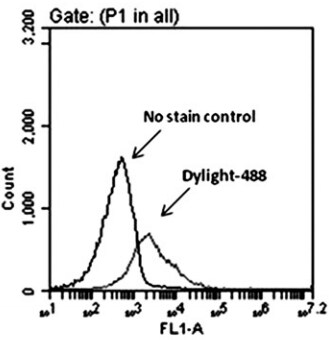
Application: Flow CytometrySample Tested:Species: HumanVerified Customer | Posted 08/11/2016Representative dot plots of Mas receptor expression in CD34 cells. Right shift indicates the surface expression of Mas receptor
There are no reviews that match your criteria.
Protocols
View specific protocols for Mas Antibody - BSA Free (NLS1531):
Mas Antibody:
Immunohistochemistry
1. Prepare tissue with formalin fixation and by embedding it in paraffin wax.
2. Make 4-um sections and place on pre-cleaned and charged microscope slides.
3. Heat in a tissue-drying oven for 45 minutes at 60 degrees Celcius.
4. Deparaffinize the tissues by wash drying the slides in 3 changes of xylene approximately 5 minutes each @ RT.
5. Rehydrate the tissues by washing the slides in 3 changes of 100% alcohol approximately 3 minutes each @ RT.
6. Wash the slides in 2 changes of 95% alcohol approximately 3 minutes each @ RT.
7. Wash the slides in 1 change of 80% alcohol approximately 3 minutes @ RT.
8. Rinse the slides in gentle running distilled water approximately 5 minutes @ RT.
9. Perform antigen retrieval by steaming the slides in 0.01M sodium citrate buffer (pH 6.0) @ 99-100 degrees Celcius for 20 minutes.
10. Remove the slides from the heat and let stand in buffer @ RT for 20 minutes.
11. Rinse the slides in 1X TBS-T for 1 minute @ RT.
**Do not allow the tissues to dry at any time during the staining procedure**
12. Begin the immunostaining by applying a universal protein block approximately 20 minutes @ RT.
13. Drain protein block from the slides and apply the diluted primary antibody approximately 45 minutes @ RT.
14. Rinse the slide in 1X TBS-T approximately 1 minute @ RT.
15. Apply a biotinylated anti-rabbit IgG (H+L) secondary approximately 30 minutes @ RT.
16. Rinse the slide in 1X TBS-T approximately 1 minute at RT.
17. Apply an alkaline phosphatase steptavidin approximately 30 minutes at RT.
18. Rinse the slide in 1X TBS-T approximately 1 minute at RT.
19. Apply an alkaline phosphatase chromagen substrate approximately 30 minutes at RT.
20. Rinse the slide in distilled water approximately 1 minute @ RT.
**This method should only be used if the chromagen substrate is alcohol insoluble (ie: Vector Red, DAB)**
21. Dehydrate the tissue by washing the slides in 2 changes of 80% alcohol approximately 1 minute each @ RT.
22. Wash the slides in 2 changes of 95% alcohol approximately 1 minute each @ RT.
23. Wash the slides in 3 changes of 100% alcohol approximately 1 minute each @ RT.
24. Wash the slides in 3 changes of xyleneapproximately 1 minute each @ RT.
25. Apply cover slip.
Immunohistochemistry
1. Prepare tissue with formalin fixation and by embedding it in paraffin wax.
2. Make 4-um sections and place on pre-cleaned and charged microscope slides.
3. Heat in a tissue-drying oven for 45 minutes at 60 degrees Celcius.
4. Deparaffinize the tissues by wash drying the slides in 3 changes of xylene approximately 5 minutes each @ RT.
5. Rehydrate the tissues by washing the slides in 3 changes of 100% alcohol approximately 3 minutes each @ RT.
6. Wash the slides in 2 changes of 95% alcohol approximately 3 minutes each @ RT.
7. Wash the slides in 1 change of 80% alcohol approximately 3 minutes @ RT.
8. Rinse the slides in gentle running distilled water approximately 5 minutes @ RT.
9. Perform antigen retrieval by steaming the slides in 0.01M sodium citrate buffer (pH 6.0) @ 99-100 degrees Celcius for 20 minutes.
10. Remove the slides from the heat and let stand in buffer @ RT for 20 minutes.
11. Rinse the slides in 1X TBS-T for 1 minute @ RT.
**Do not allow the tissues to dry at any time during the staining procedure**
12. Begin the immunostaining by applying a universal protein block approximately 20 minutes @ RT.
13. Drain protein block from the slides and apply the diluted primary antibody approximately 45 minutes @ RT.
14. Rinse the slide in 1X TBS-T approximately 1 minute @ RT.
15. Apply a biotinylated anti-rabbit IgG (H+L) secondary approximately 30 minutes @ RT.
16. Rinse the slide in 1X TBS-T approximately 1 minute at RT.
17. Apply an alkaline phosphatase steptavidin approximately 30 minutes at RT.
18. Rinse the slide in 1X TBS-T approximately 1 minute at RT.
19. Apply an alkaline phosphatase chromagen substrate approximately 30 minutes at RT.
20. Rinse the slide in distilled water approximately 1 minute @ RT.
**This method should only be used if the chromagen substrate is alcohol insoluble (ie: Vector Red, DAB)**
21. Dehydrate the tissue by washing the slides in 2 changes of 80% alcohol approximately 1 minute each @ RT.
22. Wash the slides in 2 changes of 95% alcohol approximately 1 minute each @ RT.
23. Wash the slides in 3 changes of 100% alcohol approximately 1 minute each @ RT.
24. Wash the slides in 3 changes of xyleneapproximately 1 minute each @ RT.
25. Apply cover slip.
Find general support by application which include: protocols, troubleshooting, illustrated assays, videos and webinars.
- 7-Amino Actinomycin D (7-AAD) Cell Viability Flow Cytometry Protocol
- Antigen Retrieval Protocol (PIER)
- Antigen Retrieval for Frozen Sections Protocol
- Appropriate Fixation of IHC/ICC Samples
- Cellular Response to Hypoxia Protocols
- Chromogenic IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Chromogenic Immunohistochemistry Staining of Frozen Tissue
- ClariTSA™ Fluorophore Kits
- Detection & Visualization of Antibody Binding
- Extracellular Membrane Flow Cytometry Protocol
- Flow Cytometry Protocol for Cell Surface Markers
- Flow Cytometry Protocol for Staining Membrane Associated Proteins
- Flow Cytometry Staining Protocols
- Flow Cytometry Troubleshooting Guide
- Fluorescent IHC Staining of Frozen Tissue Protocol
- Graphic Protocol for Heat-induced Epitope Retrieval
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Graphic Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- ICC Cell Smear Protocol for Suspension Cells
- ICC Immunocytochemistry Protocol Videos
- ICC for Adherent Cells
- IHC Sample Preparation (Frozen sections vs Paraffin)
- Immunocytochemistry (ICC) Protocol
- Immunocytochemistry Troubleshooting
- Immunofluorescence of Organoids Embedded in Cultrex Basement Membrane Extract
- Immunofluorescent IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Immunohistochemistry (IHC) and Immunocytochemistry (ICC) Protocols
- Immunohistochemistry Frozen Troubleshooting
- Immunohistochemistry Paraffin Troubleshooting
- Intracellular Flow Cytometry Protocol Using Alcohol (Methanol)
- Intracellular Flow Cytometry Protocol Using Detergents
- Intracellular Nuclear Staining Flow Cytometry Protocol Using Detergents
- Intracellular Staining Flow Cytometry Protocol Using Alcohol Permeabilization
- Intracellular Staining Flow Cytometry Protocol Using Detergents to Permeabilize Cells
- Preparing Samples for IHC/ICC Experiments
- Preventing Non-Specific Staining (Non-Specific Binding)
- Primary Antibody Selection & Optimization
- Propidium Iodide Cell Viability Flow Cytometry Protocol
- Protocol for Heat-Induced Epitope Retrieval (HIER)
- Protocol for Liperfluo
- Protocol for Making a 4% Formaldehyde Solution in PBS
- Protocol for VisUCyte™ HRP Polymer Detection Reagent
- Protocol for the Characterization of Human Th22 Cells
- Protocol for the Characterization of Human Th9 Cells
- Protocol for the Fluorescent ICC Staining of Cell Smears - Graphic
- Protocol for the Fluorescent ICC Staining of Cultured Cells on Coverslips - Graphic
- Protocol for the Preparation & Fixation of Cells on Coverslips
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections - Graphic
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections - Graphic
- Protocol for the Preparation and Fluorescent ICC Staining of Cells on Coverslips
- Protocol for the Preparation and Fluorescent ICC Staining of Non-adherent Cells
- Protocol for the Preparation and Fluorescent ICC Staining of Stem Cells on Coverslips
- Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- Protocol for the Preparation of a Cell Smear for Non-adherent Cell ICC - Graphic
- Protocol: Annexin V and PI Staining by Flow Cytometry
- Protocol: Annexin V and PI Staining for Apoptosis by Flow Cytometry
- TUNEL and Active Caspase-3 Detection by IHC/ICC Protocol
- The Importance of IHC/ICC Controls
- Troubleshooting Guide: Fluorokine Flow Cytometry Kits
- Troubleshooting Guide: Immunohistochemistry
- View all Protocols, Troubleshooting, Illustrated assays and Webinars
Loading...

![Immunohistochemistry-Paraffin: Mas Antibody - BSA Free [NLS1531] Immunohistochemistry-Paraffin: Mas Antibody - BSA Free [NLS1531]](https://resources.rndsystems.com/images/products/Mas-Antibody-Immunohistochemistry-Paraffin-NLS1531-img0003.jpg)
![Immunohistochemistry-Paraffin: Mas Antibody - BSA Free [NLS1531] Immunohistochemistry-Paraffin: Mas Antibody - BSA Free [NLS1531]](https://resources.rndsystems.com/images/products/Mas-Antibody-Immunohistochemistry-Paraffin-NLS1531-img0002.jpg)
![Immunohistochemistry: Mas Antibody - BSA Free [NLS1531] - Mas Antibody - BSA Free](https://resources.rndsystems.com/images/products/nls1531_rabbit-polyclonal-mas-antibody-imgenex-img-71590-271220231253318.jpg)
![Immunocytochemistry/ Immunofluorescence: Mas Antibody - BSA Free [NLS1531] - Mas Antibody - BSA Free](https://resources.rndsystems.com/images/products/nls1531_rabbit-polyclonal-mas-antibody-imgenex-img-71590-31020241517130.jpg)
![Immunocytochemistry/ Immunofluorescence: Mas Antibody - BSA Free [NLS1531] - Mas Antibody - BSA Free](https://resources.rndsystems.com/images/products/nls1531_rabbit-polyclonal-mas-antibody-imgenex-img-71590-310202416121931.jpg)
![Immunohistochemistry: Mas Antibody - BSA Free [NLS1531] - Mas Antibody - BSA Free](https://resources.rndsystems.com/images/products/nls1531_rabbit-polyclonal-mas-antibody-imgenex-img-71590-31020241612234.jpg)