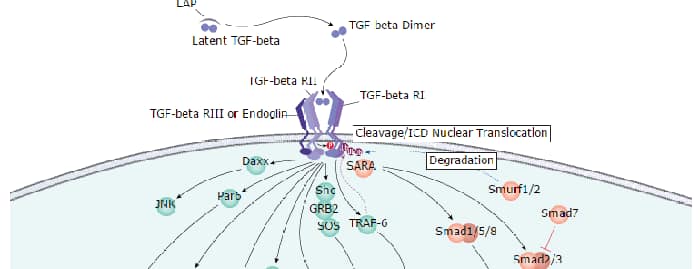
TGF-beta RII is a membrane bound serine/threonine kinase. Upon ligand binding, TGF-beta RII interacts with TGF-beta RI to form the heteromeric signaling complex thattransduces TGF beta signals. A splice variant of the type II receptor, TGF-beta RIIb, containing a 25 amino acid residue insertion near the Nterminus of the mature protein has also been described.
Human TGF‑ beta RII Antibody
R&D Systems | Catalog # AF-241-NA


Key Product Details
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Ile24-Asp159
Accession # P37173.2
Specificity
Clonality
Host
Isotype
Endotoxin Level
Scientific Data Images for Human TGF‑ beta RII Antibody
Detection of Human TGF‑ beta RII by Western Blot.
Western blot shows lysates of HepG2 human hepatocellular carcinoma cell line, Hep3B human hepatocellular carcinoma cell line, U-118-MG human glioblastoma/astrocytoma cell line, HT1080 human fibrosarcoma cell line, and DU145 human prostate carcinoma cell line. PVDF membrane was probed with 2 µg/mL of Goat Anti-Human TGF-beta RII Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-241-NA) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF017). A specific band was detected for TGF-beta RII at approximately 75 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
Detection of TGF-beta RII in Human Lymphocytes by Flow Cytometry.
Human blood-derived lymphocytes were labeled with Goat Anti-Human TGF-beta RII Antigen Affinity-purified Poly-clonal Antibody (Catalog # AF-241-NA, filled histogram) or control antibody (AB-108-C, open histogram), followed by Allophycocyanin-conjugated Anti-Goat IgG Secondary Antibody (F0108).
TGF-beta RII in Human Pituitary.
TGF-beta RII was detected in immersion fixed paraffin-embedded sections of human pituitary using Goat Anti-Human TGF-beta RII Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-241-NA) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; (CTS008) and counterstained with hematoxylin (blue). Lower panel shows a lack of labeling if primary antibodies are omitted and tissue is stained only with secondary antibody followed by incubation with detection reagents. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
TGF‑ beta 1 Inhibition of IL‑4-dependent Cell Proliferation and Neutralization by Human TGF‑ beta RII Antibody.
Recombinant Human TGF-beta 1 (Catalog # 240-B) inhibits Recombinant Human IL-4 (Catalog # 204-IL) induced proliferation in the TF-1 human erythroleukemic cell line in a dose-dependent manner (orange line). Inhibition of Recombinant Human IL-4 (5 ng/mL) activity elicited by Recombinant Human TGF-beta 1 (0.04 ng/mL) is neutral-ized (green line) by increasing concentrations of Goat Anti- Human TGF-beta RII Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-241-NA). The ND50 is typically 10-20 µg/mL.
Detection of TGF-beta RII by Western Blot
SMAD-activation by recombinant GDF15 in myeloma cell lines.A. Phosphorylation of SMAD1/5 or SMAD2 was determined using immunoblotting in IH-1 cells treated with BMP-9 (0.5 ng/mL), activin A (25 ng/mL) or indicated concentrations of GDF15 (100–400 ng/mL) for 1 hour. B. INA-6 cells were treated with GDF15 (200 ng/mL) and the inhibitor SB431542 (0–2.5 μM) for 1 hour before immunoblotting with anti-phospho-SMAD2. C. INA-6 cells were transiently transfected with siRNAs targeting ACVR1B/ALK4, ACVR1C/ALK7, TGFBR1/ALK5 and a non-targeting control siRNA. Two days after transfection the cells were treated with GDF15 (200 ng/mL) for 1 hour before immunoblotting with anti-phospho-SMAD2. D. Knock-down of receptors by siRNA in cells used in (C) as shown by QRT-PCR. Gene expression was calculated with the comparative delta Ct-method with GAPDH as housekeeping gene. The error bars indicate SEM of three independent experiments. Asterisks above bars indicate the degree of significance for downregulation of each gene compared to control (*, P≤0.05; **, P≤0.01; and ***, P≤0.001). E. INA-6 cells were treated with GDF15 (100 ng/mL) and a neutralizing TGFBR2 antibody (10–15 μM) for 1 hour before immunoblotting with anti-phospho-SMAD2. F. INA-6 cells were treated with GDF15 (100 ng/mL) and the indicated soluble receptors (5 μg/mL for all except endoglin, which was 1 μg/mL) for 1 hour before immunoblotting with anti-phospho-SMAD2. Antibody staining towards GAPDH was used as loading control for all Western blots. The experiments were performed 2–3 times each. GDF15 used in this figure was from R&D Systems, Lot# EHF1713081. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29161287), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of TGF-beta RII by Flow Cytometry
Blockade of transforming growth factor (TGF)-beta I-III did in part prevent regulatory T cells (Treg) induction. (A) CFSE-labeled CD4+CD25- T cells were cocultured with platelets in the ratio of 1:50 and were stimulated with anti-CD3 mAb (0.5 µg/mL) and anti-CD28 mAb (1.0 µg/mL) in the presence of either anti-TGF-beta I-III (10 µg/mL) or anti-TGF-beta receptor II (10 µg/mL) antibodies. Antibodies were added at day 0. The expression of Foxp3 and GARP and cell proliferation were determined on day 3 via flow cytometry. (B) Production of IL-2 and IFN-gamma was assessed by intracellular flow cytometry on day 6. The graphs show cells cultured in the presence of platelets normalized to CD4+CD25− T cells without platelets. Dot plots show one representative result of 10 independent experiments (n = 10, box and whiskers, medians ± min/max, * p < 0.05, ** p ≤ 0.01, *** p ≤ 0.001, and n.s. determined by one-way ANOVA). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/33291452), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of TGF-beta RII by Flow Cytometry
Combining blockade of TGF-beta signaling and GARP led to a complete inhibition of platelet effects. (A) CFSE-labeled CD4+CD25− T cells were cocultured with platelets in the ratio of 1:50 and were stimulated with anti-CD3 mAb (0.5 µg/mL) and anti-CD28 mAb (1.0 µg/mL). CD4+CD25− T cells were incubated for 15 min with TGF-beta receptor II (10 µg/mL) antibody prior to coculture, as indicated. Excess antibody was removed. Pre-treated CD4+CD25− T cells were cultured in the presence of either anti-TGF-beta I–III (10 µg/mL) and/or anti-GARP Ab (10 µg/mL) antibodies. Antibodies were added at day 0. The expression of Foxp3, GARP and cell proliferation were determined on day 3 via flow cytometry. (B) Production of IL-2 and IFN-gamma was assessed by intracellular flow cytometry on day 6. The graphs show cells cultured in the presence of platelets normalized to CD4+CD25− T cells without platelets. Dot plots show 1 representative result of 10 independent experiments (n = 3, means ± SD, * p < 0.05, ** p ≤ 0.01, and n.s. determined by one-way ANOVA). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/33291452), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Human TGF‑ beta RII Antibody
CyTOF-ready
Flow Cytometry
Sample: Human blood-derived lymphocytes
Immunohistochemistry
Sample: Immersion fixed paraffin-embedded sections of human pituitary
Western Blot
Sample: HepG2 human hepatocellular carcinoma cell line, Hep3B human hepatocellular carcinoma cell line, U‑118‑MG human glioblastoma/astrocytoma cell line, HT1080 human fibrosarcoma cell line, and DU145 human prostate carcinoma cell line
Neutralization
Human TGF-beta RII Sandwich Immunoassay
Flow Cytometry Panel Builder
Bio-Techne Knows Flow Cytometry
Save time and reduce costly mistakes by quickly finding compatible reagents using the Panel Builder Tool.
Advanced Features
- Spectra Viewer - Custom analysis of spectra from multiple fluorochromes
- Spillover Popups - Visualize the spectra of individual fluorochromes
- Antigen Density Selector - Match fluorochrome brightness with antigen density
Formulation, Preparation, and Storage
Purification
Reconstitution
Reconstitute at 0.2 mg/mL in sterile PBS. For liquid material, refer to CoA for concentration.
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Calculators
Background: TGF-beta RII
Long Name
Alternate Names
Gene Symbol
UniProt
Additional TGF-beta RII Products
Product Documents for Human TGF‑ beta RII Antibody
Product Specific Notices for Human TGF‑ beta RII Antibody
For research use only
Related Research Areas
Citations for Human TGF‑ beta RII Antibody
Customer Reviews for Human TGF‑ beta RII Antibody
There are currently no reviews for this product. Be the first to review Human TGF‑ beta RII Antibody and earn rewards!
Have you used Human TGF‑ beta RII Antibody?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Protocols
Find general support by application which include: protocols, troubleshooting, illustrated assays, videos and webinars.
- 7-Amino Actinomycin D (7-AAD) Cell Viability Flow Cytometry Protocol
- Antigen Retrieval Protocol (PIER)
- Antigen Retrieval for Frozen Sections Protocol
- Appropriate Fixation of IHC/ICC Samples
- Cellular Response to Hypoxia Protocols
- Chromogenic IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Chromogenic Immunohistochemistry Staining of Frozen Tissue
- Detection & Visualization of Antibody Binding
- Extracellular Membrane Flow Cytometry Protocol
- Flow Cytometry Protocol for Cell Surface Markers
- Flow Cytometry Protocol for Staining Membrane Associated Proteins
- Flow Cytometry Staining Protocols
- Flow Cytometry Troubleshooting Guide
- Fluorescent IHC Staining of Frozen Tissue Protocol
- Graphic Protocol for Heat-induced Epitope Retrieval
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Graphic Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- IHC Sample Preparation (Frozen sections vs Paraffin)
- Immunofluorescent IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Immunohistochemistry (IHC) and Immunocytochemistry (ICC) Protocols
- Immunohistochemistry Frozen Troubleshooting
- Immunohistochemistry Paraffin Troubleshooting
- Intracellular Flow Cytometry Protocol Using Alcohol (Methanol)
- Intracellular Flow Cytometry Protocol Using Detergents
- Intracellular Nuclear Staining Flow Cytometry Protocol Using Detergents
- Intracellular Staining Flow Cytometry Protocol Using Alcohol Permeabilization
- Intracellular Staining Flow Cytometry Protocol Using Detergents to Permeabilize Cells
- Preparing Samples for IHC/ICC Experiments
- Preventing Non-Specific Staining (Non-Specific Binding)
- Primary Antibody Selection & Optimization
- Propidium Iodide Cell Viability Flow Cytometry Protocol
- Protocol for Heat-Induced Epitope Retrieval (HIER)
- Protocol for Making a 4% Formaldehyde Solution in PBS
- Protocol for VisUCyte™ HRP Polymer Detection Reagent
- Protocol for the Characterization of Human Th22 Cells
- Protocol for the Characterization of Human Th9 Cells
- Protocol for the Preparation & Fixation of Cells on Coverslips
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections - Graphic
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections - Graphic
- Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- Protocol: Annexin V and PI Staining by Flow Cytometry
- Protocol: Annexin V and PI Staining for Apoptosis by Flow Cytometry
- R&D Systems Quality Control Western Blot Protocol
- TUNEL and Active Caspase-3 Detection by IHC/ICC Protocol
- The Importance of IHC/ICC Controls
- Troubleshooting Guide: Fluorokine Flow Cytometry Kits
- Troubleshooting Guide: Immunohistochemistry
- Troubleshooting Guide: Western Blot Figures
- Western Blot Conditions
- Western Blot Protocol
- Western Blot Protocol for Cell Lysates
- Western Blot Troubleshooting
- Western Blot Troubleshooting Guide
- View all Protocols, Troubleshooting, Illustrated assays and Webinars