A 803467
Tocris Bioscience | Catalog # 2976

Key Product Details
Description
Product Description
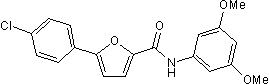
A 803467 is a selective blocker of NaV1.8 channels (IC50 values are 8, 2450, 6740, 7340 and 7380 nM for hNaV1.8, hNaV1.3, hNaV1.7, hNaV1.5 and hNaV1.2 channels respectively). Shows no significant activity against TRPV1, P2X2/3, CaV2.2 and KCNQ2/3 channels. Antinociceptive; potently attenuates mechanical allodynia in two models of neuropathic pain following i.p. administration.
Product Specifications for A 803467
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 35.78 | 100 | |
| Ethanol | 8.94 | 25 |
Preparing Stock Solutions for A 803467
The following data is based on the product molecular weight 357.79.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 2.79 mL | 13.97 mL | 27.95 mL |
| 5 mM | 0.56 mL | 2.79 mL | 5.59 mL |
| 10 mM | 0.28 mL | 1.40 mL | 2.79 mL |
| 50 mM | 0.06 mL | 0.28 mL | 0.56 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 37 publications citing the usage of this product.
- Jarvis A-803467, a potent and selective NaV1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc.Natl.Acad.Sci. 2007 PMID: 17483457
- McGaraughty A selective NaV1.8 sodium channel blocker, A-803467 [5-(4-chlorophenyl-N-(3,5-dimethoxyphenyl)furan-2-carboxamide], attenuates spinal neuronal activity in neuropathic rats. J.Pharmacol.Exp.Ther. 2008 PMID: 18089840
- Kort Discovery and biological evaluation of 5-Aryl-2-furfuramides, potent and selective blockers of the Nav1.8 sodium channel with efficacy in models of neuropathic and inflammatory pain. J.Med.Chem. 2008 PMID: 18176998
- Rush and Cummins Painful research: identification of a small-molecule inhibitor that selectively targets NaV1.8 sodium channels. Mol.Interv. 2007 PMID: 17827438
Product Documents for A 803467
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for A 803467
For research use only
Related Research Areas
Citations for A 803467
Customer Reviews for A 803467
There are currently no reviews for this product. Be the first to review A 803467 and earn rewards!
Have you used A 803467?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
