COMET™ Antibody Testing Process
IHC serves as the foundational application for spatial biology. While R&D Systems™ IHC-validated antibodies undergo a rigorous validation process before being added to the catalog, we acknowledge that antibody performance can vary across different spatial biology platforms. Ensuring optimal performance requires careful evaluation tailored to each specific application.
R&D Systems antibody validation experts, in collaboration with Lunaphore, have developed a tailored antibody testing process for Lunaphore’s COMET platform. Combining the antibody development and manufacturing expertise of R&D Systems with the spatial biology know-how of Lunaphore, ensures that Lunaphore certified antibodies are optimized for performance on COMET.
The COMET platform, from Lunaphore, a Bio-Techne brand, is a fully-automated, high-throughput, spatial biology platform that performs sequential immunofluorescence (seqIF™) through staining, imaging, and elution cycles. It offers the unique flexibility to work with off-the-shelf, non-conjugated primary antibodies. R&D Systems antibodies validated for use on COMET will have Multiplex IF (mIF) application validation and a stamp on the data image indicating they are Lunaphore Certified.
Streamline Panel Development on COMET™
The antibody testing process for COMET involves several key aspects, with a focus on assessing specificity, sensitivity, and elution efficiency of the primary antibody, using positive control tissue. R&D Systems’ Lunaphore Certified antibody testing process is shown below in Figure 1.
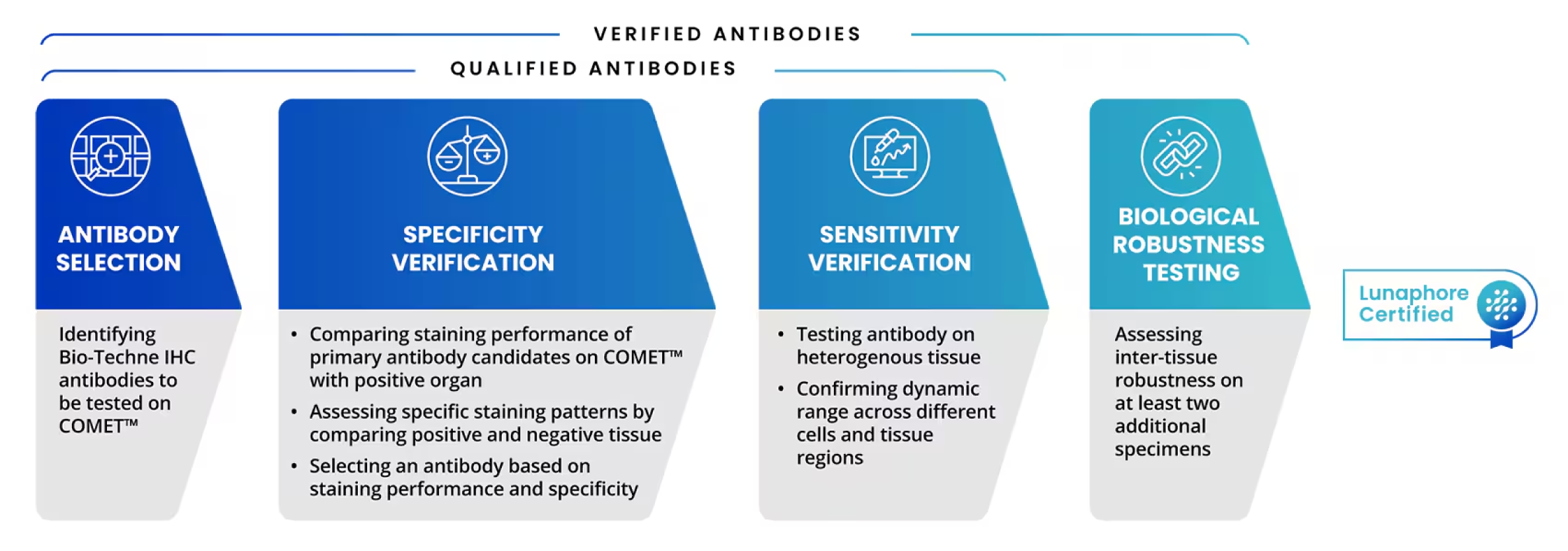
Figure 1. Steps of R&D Systems Antibody Testing Process on COMET™ that leads to Lunaphore Certified badge. Easily identify multiplex IF antibodies certified for use on COMET with our Lunaphore Certified badge. This highlights the antibodies are guaranteed to deliver high-quality and specific staining on the COMET platform. Our mIF validated antibodies are also in the Lunaphore Panel Builder and added to the expanding COMET marker database.
Featured Lunaphore Certified mIF Antibodies
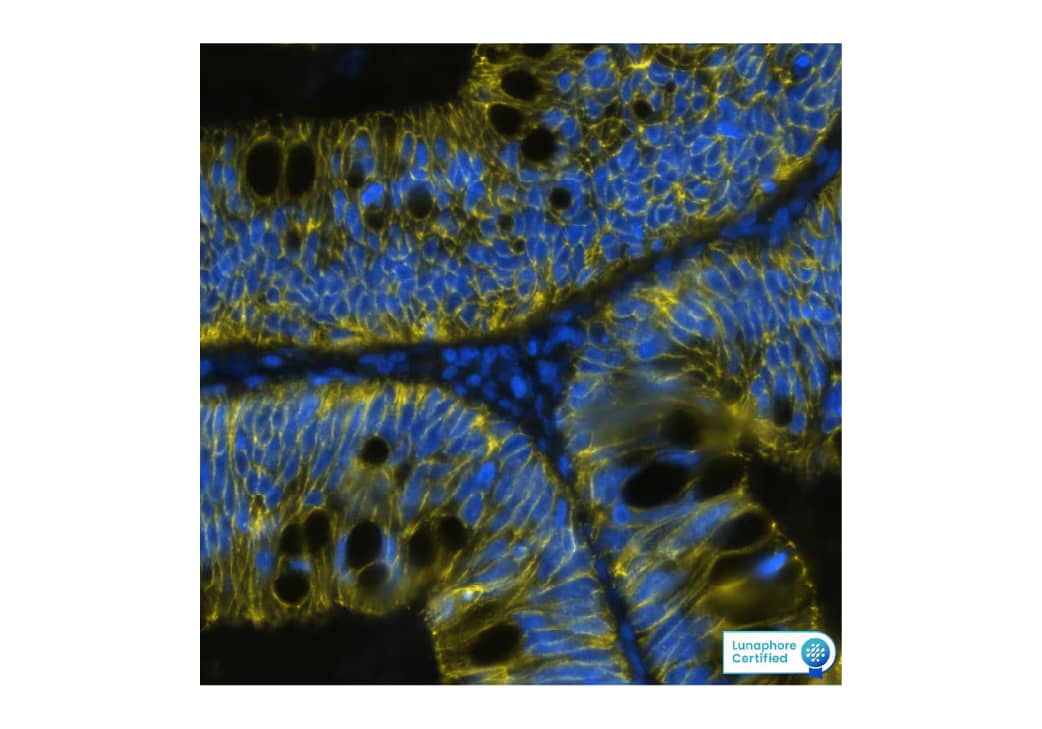
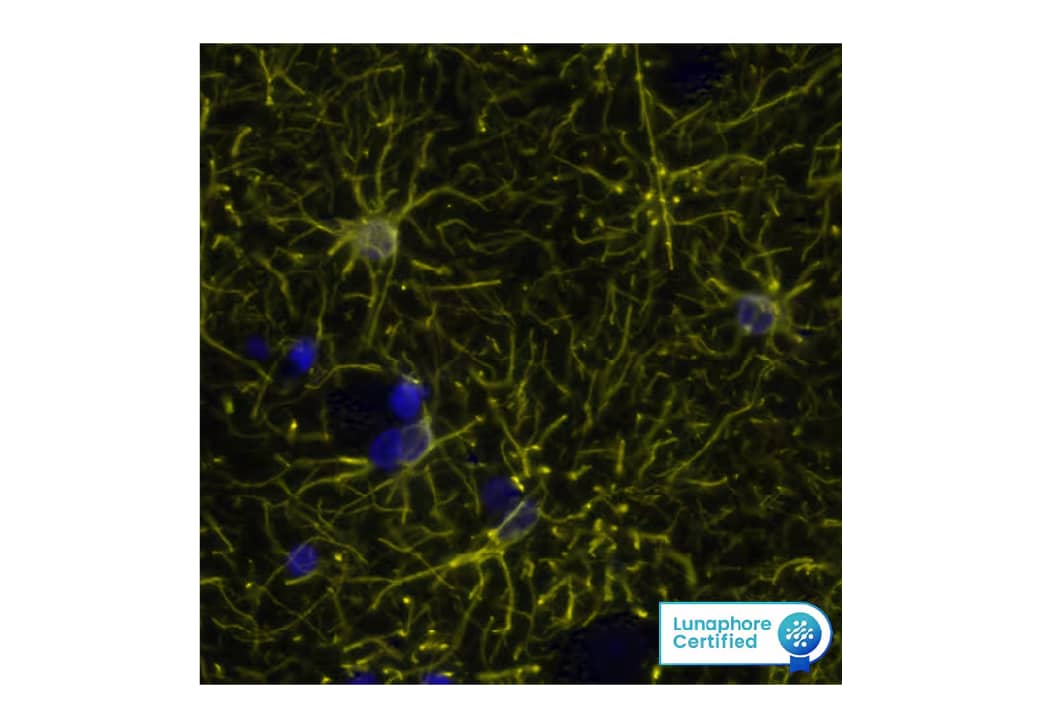
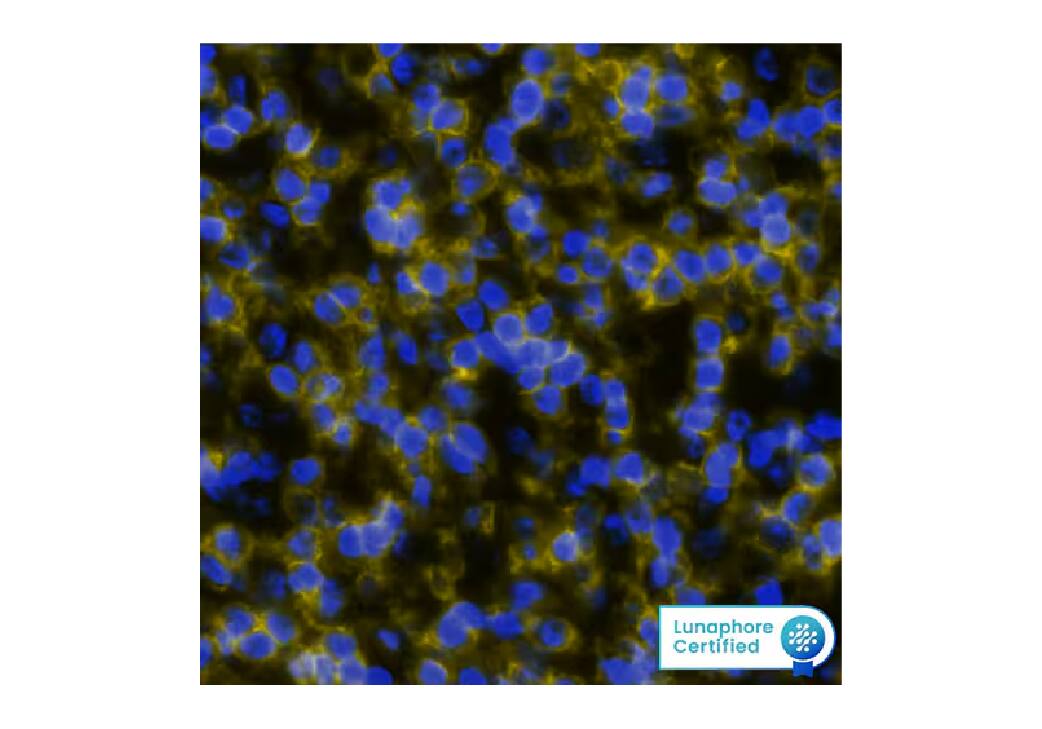
Figure 2. Detection of Cadherin in Human Colon Cancer via Multiplex Immunofluorescence staining on COMET™ using Mouse Anti-Human/Mouse Cadherin Pan Specific Monoclonal Antibody (R&D Systems, Catalog# MAB18385). Tissue was stained using the Alexa Fluor™ 647 Goat anti-Mouse IgG Secondary Antibody (Yellow; Lunaphore Catalog # DR647MS) and counterstained with DAPI (blue; Lunaphore Catalog # DR100). Specific staining was localized to the membrane.
Figure 3. Detection of GFAP in Human Brain Cortex via Multiplex Immunofluorescence staining on COMET™ using Mouse Anti-Human GFAP Monoclonal Antibody (Catalog # MAB25941) Tissue was stained using the Alexa Fluor™ 555 Goat anti-Mouse IgG Secondary Antibody (Yellow; Lunaphore Catalog # DR555MS) and counterstained with DAPI (blue; Lunaphore Catalog # DR100). Specific staining was localized to the cytoplasm.
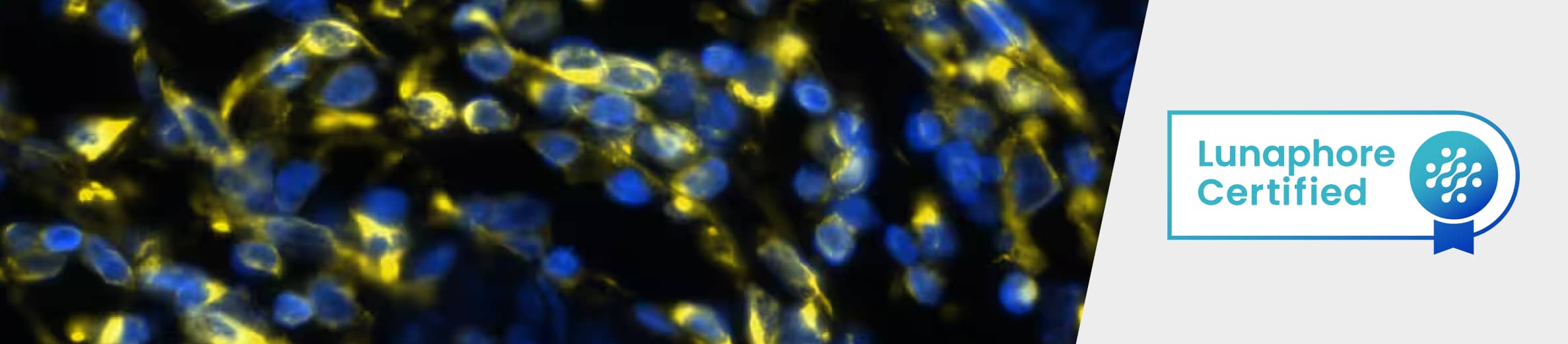
Figure 4. Detection of CD4 in Human Tonsil via Multiplex Immunofluorescence staining on COMET™ using Rabbit Anti-Human CD4 Monoclonal Antibody (R&D Systems, Catalog # MAB11560). Tissue was stained using the Alexa Fluor™ Plus 647 Goat anti-Rabbit IgG Secondary Antibody (Yellow; Lunaphore Catalog # DR647RB) and counterstained with DAPI (blue; Lunaphore Catalog # DR100). Specific staining was localized to the membrane.