APETx2
Tocris Bioscience | Catalog # 4804

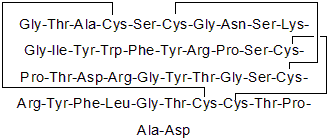
Product Description
APETx2 is an acid-sensing ion channel 3 (ASIC3) channel blocker (IC50 values are 63 and 175 nM for homomeric rat and human ASIC3 channels). Also inhibits NaV1.8 and NaV1.2 channels (IC50 values are 55 and 114 nM respectively). Demonstrates analgesic properties against acid-induced and inflammatory pain.
Product Specifications for APETx2
Molecular Weight
Formula
Storage
Purity
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solubility | Soluble to 5mg/ml in water |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 7 publications citing the usage of this product.
- Peigneur A natural point mutation changes both target selectivity and mechanism of action of sea anemone toxins. FASEB J. 2012 PMID: 22972919
- Blanchard Inhibition of voltage-gated Na(+) currents in sensory neurones by the sea anemone toxin APETx2. Br.J.Pharmacol. 2012 PMID: 21943094
- Karczewski Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br.J.Pharmacol. 2010 PMID: 20860671
- Diochot A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004 PMID: 15044953
- Xiong Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr.Opin.Pharmacol. 2008 PMID: 17945532
Product Documents for APETx2
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for APETx2
For research use only
Citations for APETx2
Customer Reviews for APETx2
There are currently no reviews for this product. Be the first to review APETx2 and earn rewards!
Have you used APETx2?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
