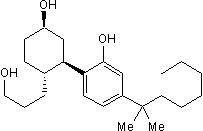
CP 55,940
Discontinued Product
Chemical Name: (-)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
Purity: ≥98%
Biological Activity
Cannabinoid agonist which is considerably more potent than Δ9-THC in both behavioral tests and receptor binding assays. Displays high and roughly equal affinity for both central and peripheral cannabinoid receptors (Ki = 0.6 - 5.0 and 0.7 - 2.6 nM at CB1 and CB2 respectively; EC50 values are 0.2, 0.3 and 5 nM at CB1, CB2 and GRP55 respectively).Technical Data
The technical data provided above is for guidance only.
For batch specific data refer to the Certificate of Analysis.
Tocris products are intended for laboratory research use only, unless stated otherwise.
Additional Information
Background References
-
CB1 Receptor Autoradiographic Characterization of the Individual Differences in Approach and Avoidance Motivation.
Laricchiuta D, Rojo ML, Rodriguez-Gaztelumendi A
PLoS ONE, 2012;7(7):e42111. -
Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors.
Xiong W, Cui T, Cheng K, Yang F, Chen SR, Willenbring D, Guan Y, Pan HL, Ren K, Xu Y, Zhang L
J. Exp. Med., 2012;209(6):1121-34. -
Corticosteroid dependent and independent effects of a cannabinoid agonist on core temperature, motor activity, and prepulse inhibition of the acoustic startle reflex in Wistar rats.
Avdesh
Psychopharmacology, 2011;:Epub ahead of print -
Evaluation of cannabinoid receptor agonists and antagonists using the guanosine-5'-O-(3-[35S]thio)-triphosphate binding assay in rat cerebellar membranes.
Griffin et al.
J.Pharmacol.Exp.Ther., 1998;285:553 -
Comparative receptor binding analyses of cannabinoid agonists and antagonists.
Thomas et al.
J.Pharmacol.Exp.Ther., 1998;285:285 -
Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats.
Wiley et al.
Neuropharmacology, 1995;34:669
Product Datasheets
Reconstitution Calculator
Molarity Calculator
Citations for CP 55,940
The citations listed below are publications that use Tocris products. Selected citations for CP 55,940 include:
54 Citations: Showing 1 - 10
-
Activity in nodose ganglia neurons after treatment with CP 55,940 and cholecystokinin.
Authors: Johnston Et al.
Physiol Rep 2018;6:e13927
-
Structure-Based Identification of Potent Natural Product Chemotypes as Cannabinoid Receptor 1 Inverse Agonists.
Authors: Pandey Et al.
Molecules 2018;23
-
Characterization of structurally novel G protein biased CB1 agonists: implications for drug development.
Authors: Ford
Pharmacol Res 2017;125:161
-
Regulation of divalent metal transporter-1 by serine phosphorylation.
Authors: Seo Et al.
Biochem J 2016;473:4243
-
Cannabinoid receptor-specific mechanisms to alleviate pain in sickle cell anemia via inhibition of mast cell activation and neurogenic inflammation.
Authors: Vincent Et al.
Haematologica 2016;101:566
-
(4-(Bis(4-fluorophenyl)methyl)piperazin-1-yl)(cyclohexyl)methanone hydrochloride (LDK1229): a new cannabinoid CB1 receptor inverse agonist from the class of benzhydryl piperazine analogs.
Authors: Mahmoud Et al.
Mol Pharmacol 2015;87:197
-
Preclinical evaluation of SMM-189, a cannabinoid receptor 2-specific inverse agonist.
Authors: Presley Et al.
Eur J Pharmacol 2015;3:e00159
-
Increasing levels of the endocannabinoid 2-AG is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease.
Authors: Mounsey Et al.
Pharmacol Res Perspect 2015;273:36
-
Flavanones from Miconia prasina.
Authors: Tarawneh Et al.
Phytochem Lett 2014;7:130
-
Potential upstream regulators of cannabinoid receptor 1 signaling in prostate cancer: a Bayesian network analysis of data from a tissue microarray.
Authors: Häggström Et al.
J Biol Chem 2014;74:1107
-
The cannabinoid agonist HU-210: pseudo-irreversible discriminative stimulus effects in rhesus monkeys.
Authors: Hruba and McMahon
Br J Pharmacol 2014;727:35
-
Prefrontal deficits in a murine model overexpressing the down syndrome candidate gene dyrk1a.
Authors: Thomazeau Et al.
J Neurosci 2014;34:1138
-
Analgesic effect of a mixed T-type channel inhibitor/CB2 receptor agonist.
Authors: Gadotti Et al.
Mol Pain 2013;9:32
-
CB(1) receptor allosteric modulators display both agonist and signaling pathway specificity.
Authors: Baillie Et al.
Mol Pharmacol 2013;83:322
-
Long-term CB1 receptor blockade enhances vulnerability to anxiogenic-like effects of cannabinoids.
Authors: Tambaro Et al.
Neuropharmacology 2013;70:268
-
Mastering tricyclic ring systems for desirable functional cannabinoid activity.
Authors: Petrov Et al.
Eur J Med Chem 2013;69:881
-
CB1 and CB2 receptors are novel molecular targets for tamox. and 4OH-Tamoxifen.
Authors: Prather Et al.
Biochem Biophys Res Commun 2013;441:339
-
Phencyclidine-induced social withdrawal results from deficient stimulation of cannabinoid CB1 receptors: implications for schizophrenia.
Authors: Seillier Et al.
Eur J Pharmacol 2013;38:1816
-
Novel insights into CB1 cannabinoid receptor signaling: a key interaction identified between the extracellular-3 loop and transmembrane helix 2.
Authors: Marcu Et al.
J Pharmacol Exp Ther 2013;345:189
-
Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors.
Authors: Rajasekaran Et al.
Toxicol Appl Pharmacol 2013;269:100
-
Real-time characterization of cannabinoid receptor 1 (CB1 ) allosteric modulators reveals novel mechanism of action.
Authors: Cawston Et al.
Br J Pharmacol 2013;170:893
-
Characterization of cannabinoid receptor ligands in tissues natively expressing cannabinoid CB2 receptors.
Authors: Marini Et al.
Br J Pharmacol 2013;169:887
-
Working memory- and anxiety-related behavioral effects of repeated nicotine as a stressor: the role of cannabinoid receptors.
Authors: Hayase
BMC Neurosci 2013;14:20
-
Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity.
Authors: Brents Et al.
Biochem Pharmacol 2012;83:952
-
CB1 receptor autoradiographic characterization of the individual differences in approach and avoidance motivation.
Authors: Laricchiuta Et al.
PLoS One 2012;7:e42111
-
Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands.
Authors: Chimalakonda Et al.
Drug Metab Dispos 2012;40:2174
-
AM630 behaves as a protean ligand at the human cannabinoid CB2 receptor.
Authors: Bolognini Et al.
Br J Pharmacol 2012;165:2561
-
A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury.
Authors: Horváth Et al.
J Physiol 2012;165:2462
-
Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness.
Authors: Cain Et al.
Br J Haematol 2012;156:535
-
Functional characterization and analgesic effects of mixed cannabinoid receptor/T-type channel ligands.
Authors: You Et al.
Mol Pain 2011;7:89
-
Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity.
Authors: Brents Et al.
PLoS One 2011;6:e21917
-
Convulsant doses of a DA D1 receptor agonist result in Erk-dependent increases in Zif268 and Arc/Arg3.1 expression in mouse dentate gyrus.
Authors: Gangarossa Et al.
PLoS One 2011;6:e19415
-
Cannabinoids attenuate hippocampal γ oscillations by suppressing excitatory synaptic input onto CA3 pyramidal neurons and fast spiking basket cells.
Authors: Holderith Et al.
Br J Pharmacol 2011;589:4921
-
Symptom-relieving and neuroprotective effects of the phytocannabinoid △9-THCV in animal models of Parkinson's disease.
Authors: García Et al.
J Cell Mol Med 2011;163:1495
-
A putative 'pre-nervous' endocannabinoid system in early echinoderm development.
Authors: Buznikov Et al.
J Biol Chem 2010;32:43101
-
Location, structure, and dynamics of the synthetic cannabinoid ligand CP-55,940 in lipid bilayers.
Authors: Kimura Et al.
Biophys J 2009;96:4916
-
Genetic and pharmacological manipulations of the CB(1) receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice.
Authors: Vinod Et al.
Synapse 2008;62:574
-
Unique agonist-bound cannabinoid CB1 receptor conformations indicate agonist specificity in signaling.
Authors: Georgieva Et al.
Neuropsychopharmacology 2008;581:19
-
Mapping the structural requirements in the CB1 cannabinoid receptor transmembrane helix II for signal transduction.
Authors: Kapur Et al.
J Pharmacol Exp Ther 2008;325:341
-
MDA7: a novel selective agonist for CB2 receptors that prevents allodynia in rat neuropathic pain models.
Authors: Naguib Et al.
Br J Pharmacol 2008;155:1104
-
Activation of cannabinoid receptors prevents antigen-induced asthma-like reaction in guinea pigs.
Authors: Giannini Et al.
Prostate 2008;12:2381
-
The psychoactive plant cannabinoid, Delta9-tetrahydrocannabinol, is antagonized by Delta8- and Delta9-tetrahydrocannabivarin in mice in vivo.
Authors: Pertwee Et al.
Br J Pharmacol 2007;150:586
-
S-nitrosothiols modulate G protein-coupled receptor signaling in a reversible and highly receptor-specific manner.
Authors: Kokkola Et al.
BMC Cell Biol 2005;6:21
-
Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation.
Authors: Ehrhart Et al.
J Neuroinflammation 2005;2:29
-
Evidence that the plant cannabinoid Delta9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist.
Authors: Thomas Et al.
Br J Pharmacol 2005;146:917
-
Identification of WIN55212-3 as a competitive neutral antagonist of the human cannabinoid CB2 receptor.
Authors: Savinainen Et al.
Br J Pharmacol 2005;145:636
-
The endocannabinoid 2-arachidonylglycerol decreases the immunological activation of Guinea pig mast cells: involvement of nitric oxide and eicosanoids.
Authors: Vannacci Et al.
J Pharmacol Exp Ther 2004;311:256
-
Hypersensitization of the Orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716.
Authors: Hilairet Et al.
Exp Neurol 2003;278:23731
-
An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors.
Authors: Savinainen Et al.
Br J Pharmacol 2003;140:1451
-
Cannabinoid receptor-independent inhibition by cannabinoid agonists of the peripheral 5-HT3 receptor-mediated von Bezold-Jarisch reflex.
Authors: Godlewski Et al.
Br J Pharmacol 2003;138:767
-
Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site.
Authors: Barann Et al.
Br J Pharmacol 2002;137:589
-
Pharmacological analysis of cannabinoid receptor activity in the rat vas deferens.
Authors: Christopoulos Et al.
Br J Pharmacol 2001;132:1281
-
The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways.
Authors: Tucker Et al.
Br J Pharmacol 2001;132:1127
-
The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery.
Authors: White and Hiley
Br J Pharmacol 1998;125:533
FAQs
No product specific FAQs exist for this product, however you may
View all Small Molecule FAQsReviews for CP 55,940
There are currently no reviews for this product. Be the first to review CP 55,940 and earn rewards!
Have you used CP 55,940?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image