GMP Human AB Serum
GMP Human AB Serum Summary
Small donor, GMP-compliant human AB serum designed for clinical cell therapy workflows.Key Benefits
• Used to formulate 2 liters of GMP Human T Cell Media at 5% supplementation
• cGMP, Xeno-free, and testing is compliant with requirements outlined in 21 CFR 610.40
• Extensive viral testing done at the donor and pool levels
• Small donor pool derived material
• Heat inactivation protocol available
• Have questions? See our FAQs
GMP Human AB Serum is collected from a maximum of sixteen (16) healthy male donors of the AB serotype at FDA-licensed facilities in the United States. This material is defibrinated from source plasma AB using therapeutic grade recombinant human Thrombin. All donor units are tested for viral markers and found to be non-reactive or negative per 21 CFR 610.40 guidelines. GMP Human AB Serum is 0.1 μm sterile filtered, manufactured xeno-free, and does not contain non-human animal-derived components. GMP Human AB Serum is available in a 100 mL bottle format.
Kit Contents
• 100 mL Bottle of GMP Human AB Serum (HABS001-GMP-100ML).
Specifications
Product Datasheets
Scientific Data
 View Larger
View Larger
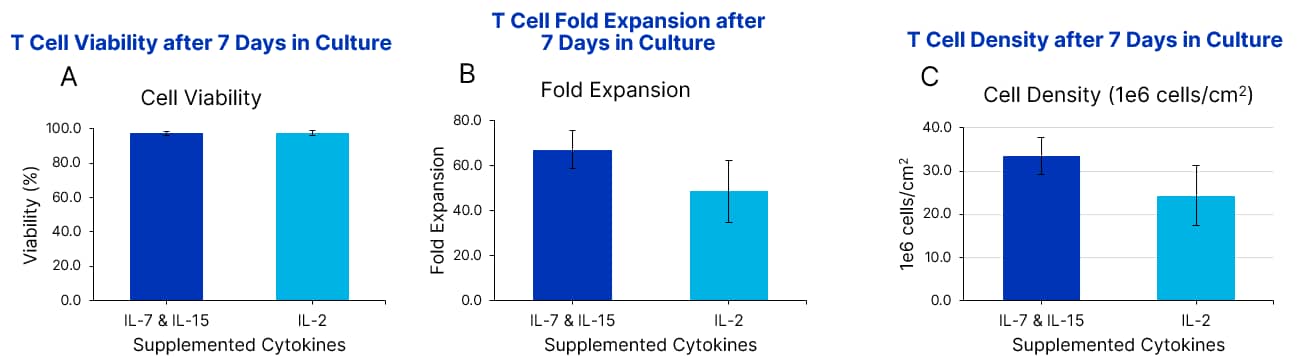
GMP Human AB Serum supports high expansion and viability in T cell cultures Xeno-Free GMP Human T Cell Media (CCM038-GMP-1L) was formulated with 5% human AB Serum (HABS001-GMP-100ML) and 10 ng/mL of IL-7 (BT-007-GMP) and IL-15 (BT-015-GMP) OR IL-2 (BT-002-GMP) cytokines. Using fresh complete media, T cells were expanded with a T cell activator in a G-Rex 6M bioreactor for 7 days. T cell viability (A), fold expansion (B), and cell density (C) after 7 days in culture are shown. Data is the average of 3 purified T cell donors with error bars ± SD.
 View Larger
View Larger
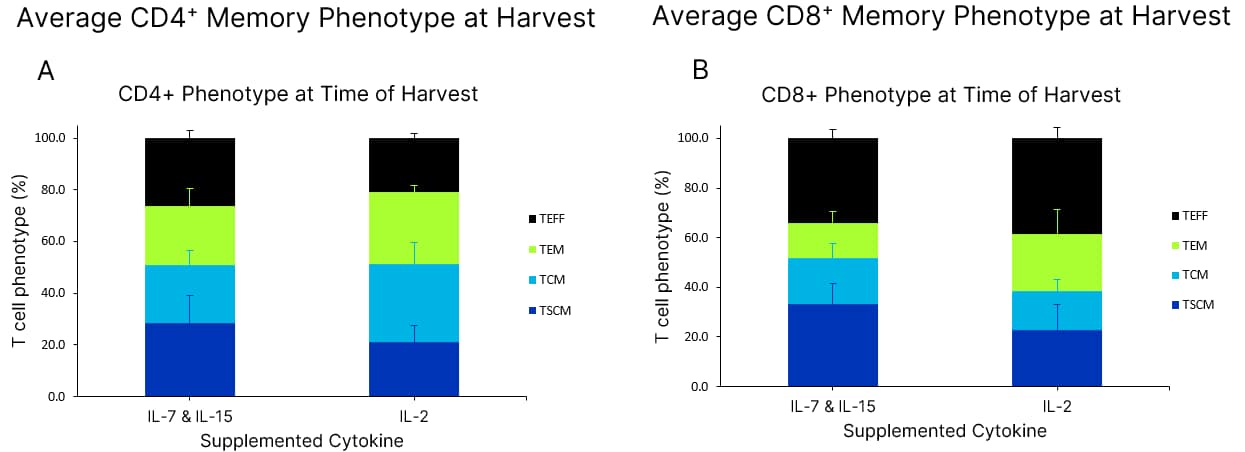
T cell phenotype when cultured with GMP Human AB Serum Xeno-Free GMP Human T Cell Media (CCM038-GMP-1L) was formulated with 5% human AB Serum (HABS001-GMP-100ML) and 10 ng/mL of IL-7 (BT-007-GMP) and IL-15 (BT-015-GMP) OR IL-2 (BT-002-GMP) cytokines. Using fresh complete media, T cells were expanded with a T cell activator in a G-Rex 6M bioreactor for 7 days. T cell memory phenotype was analyzed for either the CD4+ (A) or CD8+ (B) T cell population after 7 days in culture. T cell phenotype is gated on CD45RA and CCR7. Data is the average of 3 purified T cell donors with error bars ± SD.
 View Larger
View Larger
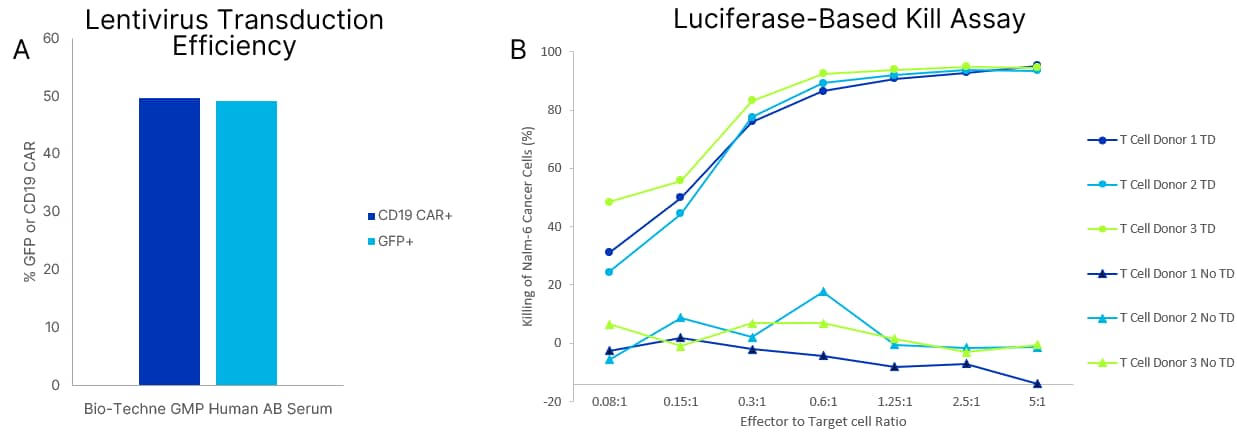
Transduction of T cells cultured with GMP Human AB Serum T cells from 3 different donors were cultured in Bio-Techne’s CCM038 media supplemented with 10 ng/mL IL-7 and IL-15 and 5% GMP Human AB Serum. Cells were thawed, plated in a 6M G-rex and transduced one day post-thaw via lentivirus (EF1a-CD19-DHFReGFP, MOI of 5). The percentage of GFP and CD19 CAR expressed in T cells is shown after 8 total days in culture. Transduction efficiency is shown as a combination of 3 donors (A). A luciferase-base kill assay was performed to measure T cell killing of Nalm-6 cancer cells. T cells cultured in A were cryopreserved prior to kill assay. T cells were thawed and plated with Nalm-6 cells at effector: target ratios ranging from 5:1 to 0.08:1 and cultured for 24 hours. For transduced T cells, cells were plated based on CD19 CAR+ positive cells determined via flow cytometry prior to freezing. Data (B) shows the three T cell donors grown with GMP Human AB Serum and includes transduced samples (TD) and grow out samples (No TD). Transduced T cells grown in media containing GMP Human AB Serum showed high percentage killing of Nalm-6 cancer cells.
 View Larger
View Larger
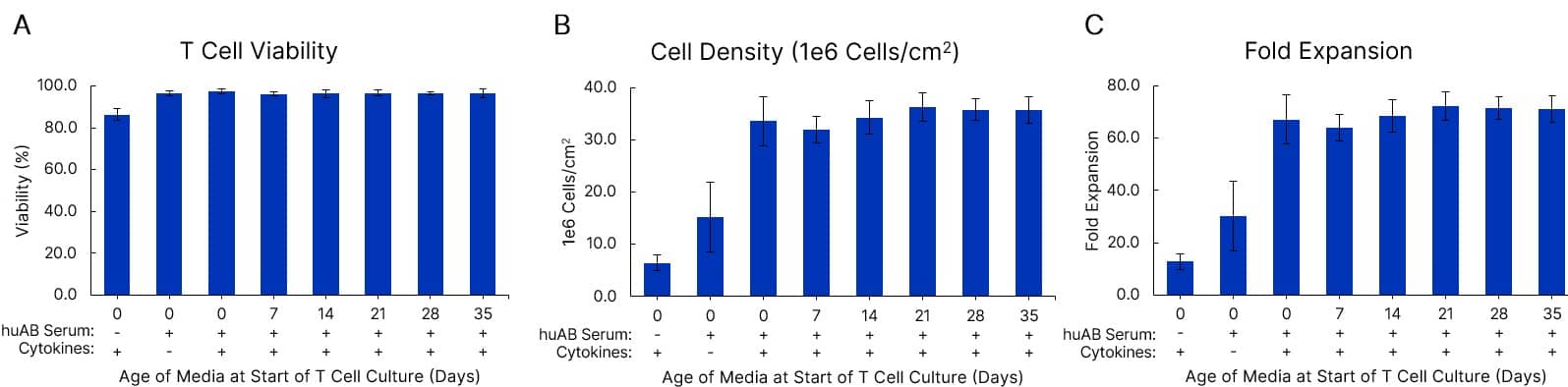
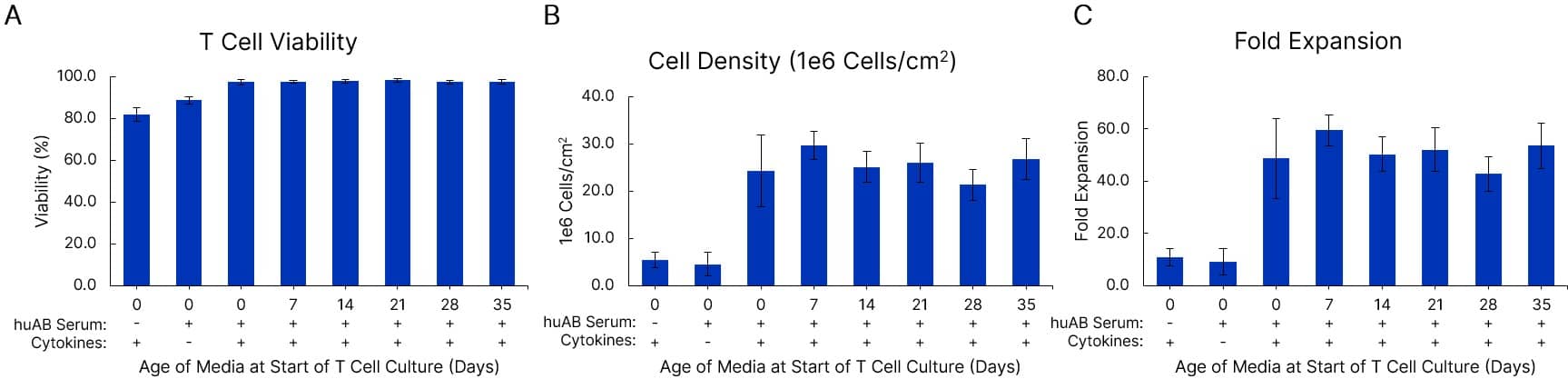
T cell viability and growth are equivalent in fresh and pre-made media stored at 4 °C for at least 5 weeks. Xeno-Free GMP Human T Cell Media (CCM038-GMP-1L) was formulated with 5% human AB Serum (HABS001-GMP-100ML) and 10 ng/mL of IL-7 (BT-007-GMP) and IL-15 (BT-015-GMP) cytokines. Media was either used fresh or stored at 2-8 °C for 7-35 days prior to use in human T cell culture. T cells were expanded with a T cell activator in a G-Rex 6M bioreactor for 7 days. T cell viability (A), cell density (B), and fold expansion (C) after 7 days in culture are shown. Data is the average of 3 purified T cell donors with error bars ± SD.
 View Larger
View Larger
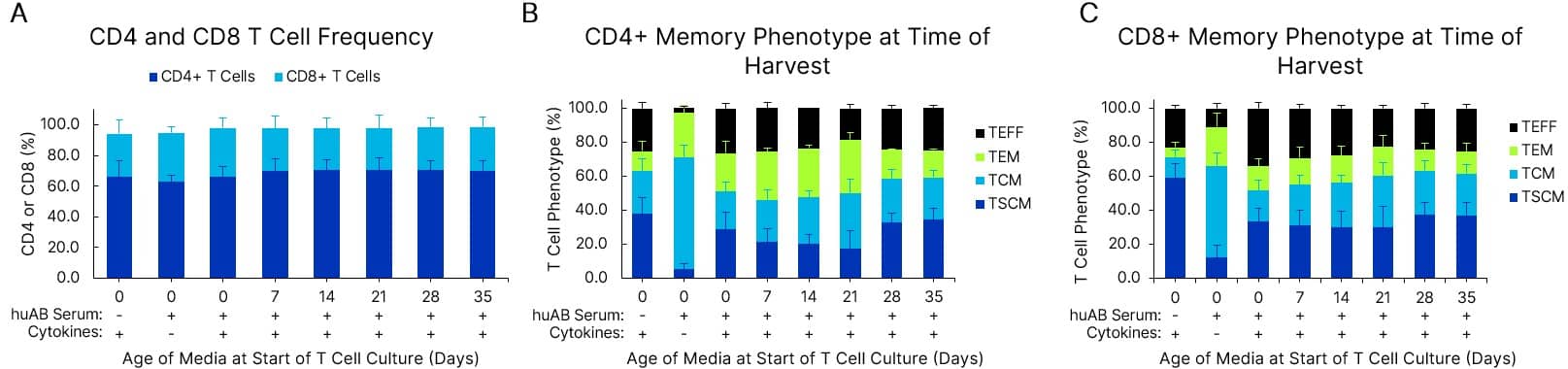
T cell phenotype is maintained in fresh and pre-made media stored at 4 °C for at least 5 weeks. Xeno-Free GMP Human T Cell Media (CCM038-GMP-1L) was formulated with 5% human AB Serum (HABS001-GMP-100ML) and 10 ng/mL of IL-7 (BT-007-GMP) and IL-15 (BT-015-GMP) cytokines. Media was either used fresh or stored at 2-8 °C for 7-35 days prior to use in human T cell culture. T cells were expanded with a T cell activator in a G-Rex 6M bioreactor for 7 days. Frequency of CD4+ or CD8+ T cells (A), CD4+ T cell phenotype (B), and CD8+ phenotype (C) after 7 days in culture are shown. T cell phenotype is gated on CD45RA and CCR7. Data is the average of 3 purified T cell donors with error bars ± SD.
 View Larger
View Larger
T cell viability and growth are equivalent in fresh and pre-made media stored at 4 °C for at least 5 weeks. Xeno-Free GMP Human T Cell Media (CCM038-GMP-1L) was formulated with 5% human AB Serum (HABS001-GMP-100ML) and 10 ng/mL of IL-2 (BT-002-GMP) cytokine. Media was either used fresh or stored at 2-8 °C for 7-35 days prior to use in human T cell culture. T cells were expanded with a T cell activator in a G-Rex 6M bioreactor for 7 days. T cell viability (A), cells density (B), and fold expansion (C) after 7 days in culture are shown. Data is the average of 3 purified T cell donors with error bars ± SD.
 View Larger
View Larger
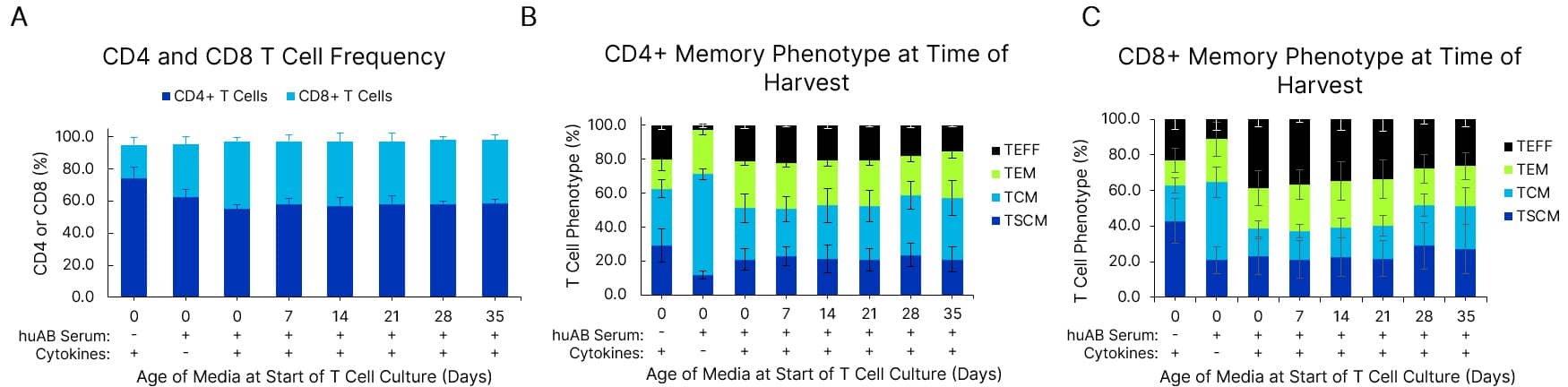
T cell phenotype is maintained in fresh and pre-made media stored at 4 °C for at least 5 weeks. Xeno-Free GMP Human T Cell Media (CCM038-GMP-1L) was formulated with 5% human AB Serum (HABS001-GMP-100ML) and 10 ng/mL of IL-2 (BT-002-GMP) cytokine. Media was either used fresh or stored at 2-8 °C for 7-35 days prior to use in human T cell culture. T cells were expanded with a T cell activator in a G-Rex 6M bioreactor for 7 days. Frequency of CD4+ or CD8+ T cells (A), CD4+ T cell phenotype (B), and CD8+ phenotype (C) after 7 days in culture are shown. T cell phenotype is gated on CD45RA and CCR7. Data is the average of 3 T cell donors with error bars ± SD.
FAQs
No product specific FAQs exist for this product, however you may
View all FAQsReviews for GMP Human AB Serum
There are currently no reviews for this product. Be the first to review GMP Human AB Serum and earn rewards!
Have you used GMP Human AB Serum?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image

