Human IL-33 Antibody Summary
Ser112-Thr270
Accession # O95760
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
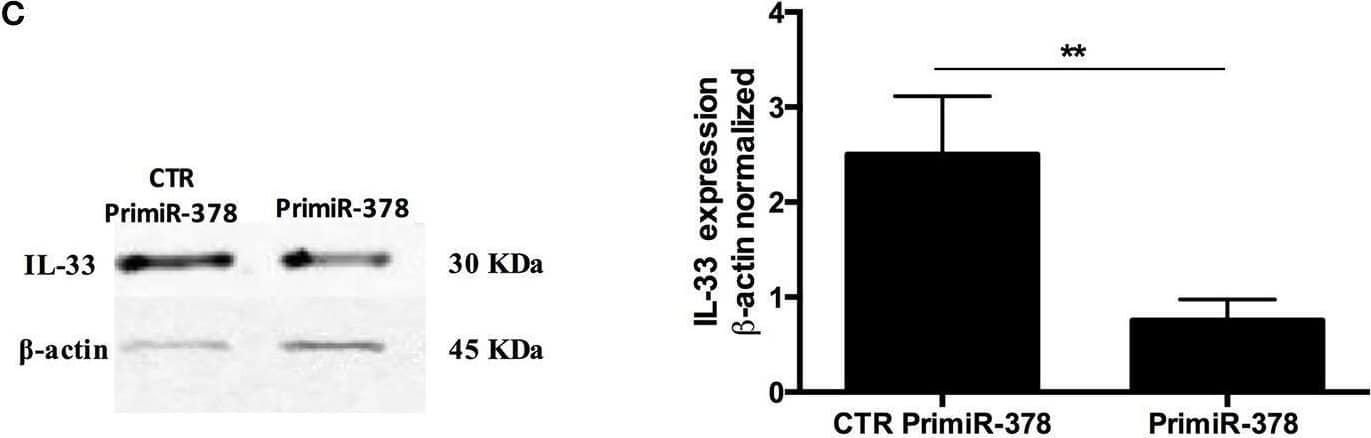
Detection of IL-33 by Western Blot Over-expression and inhibition of miR-378a-3p modulate IL-33 expression. Stable expression of miR-378a-3p (Primir-378) or inhibition of miR-378a-3p (Inhibitor), and their respective controls (CTR PrimiR-378a, CTR Inhibitor) were obtained with Lentiviral vectors in HT-29 cells. (A) MiR-378a-3p Log2 fold change, and (B) IL-33 mRNA fold change relative to CTR cell lines by qPCR, (C) IL-33 protein and beta -actin by Western Blotting (WB) and bands quantification by densitometry analysis (Fiji Software) of Control Primir-378 and Primir-378 cell lines, and (D) Control Inhibitor and Inhibitor cell lines. Four experiments by duplicated were performed. Each cell line was compared with their respective control using Paired t-test. *P = < 0.05, **P = < 0.01, ***P = < 0.001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31824476), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
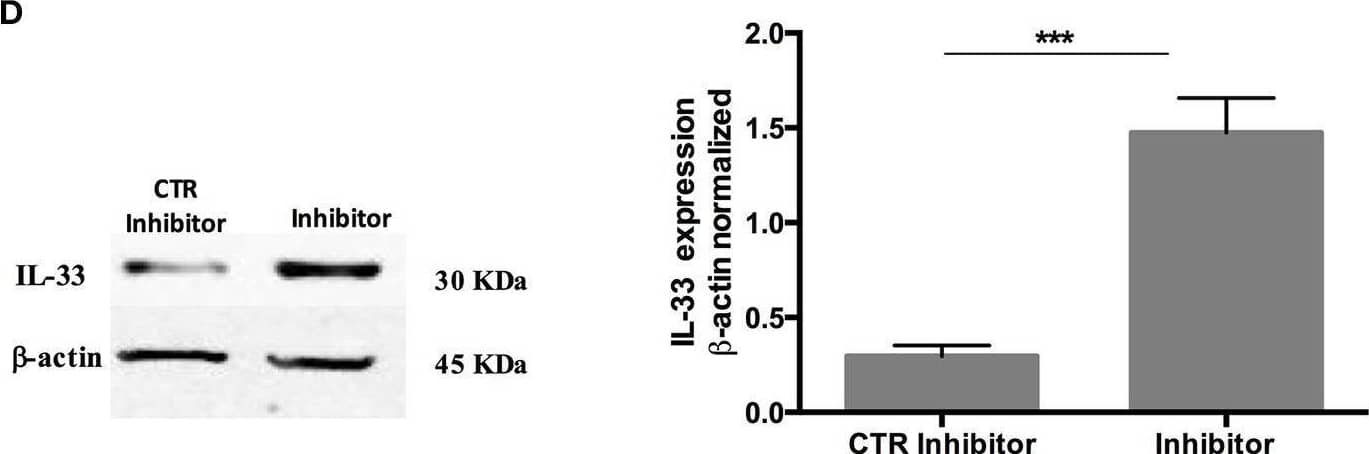
Detection of IL-33 by Western Blot Over-expression and inhibition of miR-378a-3p modulate IL-33 expression. Stable expression of miR-378a-3p (Primir-378) or inhibition of miR-378a-3p (Inhibitor), and their respective controls (CTR PrimiR-378a, CTR Inhibitor) were obtained with Lentiviral vectors in HT-29 cells. (A) MiR-378a-3p Log2 fold change, and (B) IL-33 mRNA fold change relative to CTR cell lines by qPCR, (C) IL-33 protein and beta -actin by Western Blotting (WB) and bands quantification by densitometry analysis (Fiji Software) of Control Primir-378 and Primir-378 cell lines, and (D) Control Inhibitor and Inhibitor cell lines. Four experiments by duplicated were performed. Each cell line was compared with their respective control using Paired t-test. *P = < 0.05, **P = < 0.01, ***P = < 0.001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31824476), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
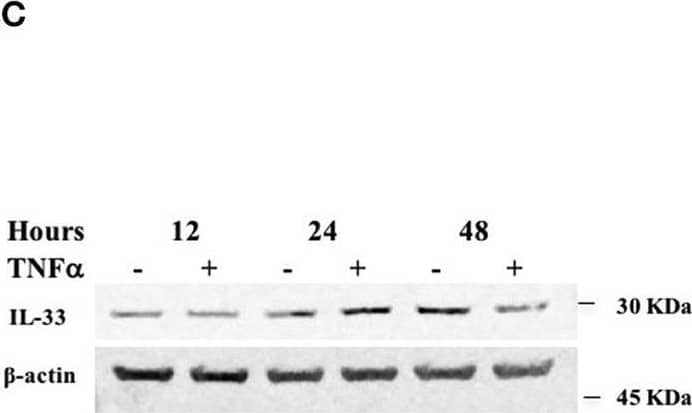
Detection of IL-33 by Western Blot TNF alpha decreases miR-378a-3p and increases IL-33 expression in colonocytes. HT-29 cell line was stimulated with 10 ng/mL human recombinant TNF alpha (hr-TNF alpha ) for 3, 6, and 24 h. Fold change relative to non-stimulated control of (A) MiR-378a-3p and (B) IL-33 mRNA. (C) IL-33 protein was measured in HT-29 cells stimulated with hr-TNF alpha 10 ng/mL 12, 24, and 48 h by WB, (D) Band quantification of IL-33 protein normalized to beta -actin. (E) Fold change relative to non-stimulated control of PPARGC1B mRNA and (F) IL-8 mRNA levels (measured as control of TNF alpha stimulus). (G) AGO1 and (H) AGO2 mRNA levels were assessed as control of miRNA-biogenesis machinery. Four experiments by duplicated were analyzed using Two-way ANOVA with Bonferroni post-test. *P < 0.05, **P = < 0.01. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31824476), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: IL-33
IL-33, also known as NF-HEV and DVS 27, is a proinflammatory protein that may also regulate gene transcription (1-3). DVS 27 was identifed as a gene that is upregulated in vasospastic cerebral arteries (1). NF-HEV was described as a nuclear factor that is preferentially expressed in the endothelial cells of high endothelial venules relative to endothelial cells from other tissues (2). IL-33 was identified based on sequence and structural homology with IL-1 family cytokines (3). IL-33 is constitutively expressed in smooth muscle and airway epithelia. It is upregulated in arterial smooth muscle, dermal fibroblasts, and keratinocytes following IL-1 alpha or IL‑1 beta stimulation (1, 3). Similar to IL-1, IL-33 can be cleaved in vitro by caspase-1, generating an N-terminal fragment that is slightly shorter than the C-terminal fragment (3, 4). The N-terminal portion of full length 32 kDa IL-33 contains a predicted bipartite nuclear localization sequence and a homeodomain-like helix-turn-helix DNA binding domain. By immunofluorescence, full length IL-33 localizes to the nucleus in HUVECs and transfectants (2). The C-terminal 18 kDa fragment, corresponding to mature IL-33, binds and triggers signaling through mast cell IL-1 R4/ST2, a receptor involved in the augmentation of Th2 cell responses (3, 5-7). A ternary signaling complex is formed by the subsequent association of IL-33 and ST2 with IL-1R AcP (8). Stimulation of Th2 polarized lymphocytes with mature IL-33 in vitro induces IL-5 and IL-13 secretion (3). In vivo administration of mature IL-33 promotes increased production of IL-5, IL-13, IgE, and IgA, as well as splenomegaly and inflammatory infiltration of mucosal tissues (3). Full length and mature human IL-33 share 52‑58% aa sequence identity with mouse and rat IL-33. Human IL-33 shares less than 20% aa sequence identity with other IL-1 family proteins.
- Onda, H. et al. (1999) J. Cereb. Blood Flow Metab. 19:1279.
- Baekkevold, E.S. et al. (2003) Am. J. Pathol. 163:69.
- Schmitz, J. et al. (2005) Immunity 23:479.
- Black, R.A. et al. (1989) J. Biol. Chem. 264:5323.
- Xu, D. et al. (1998) J. Exp. Med. 187:787.
- Lohning, M. et al. (1998) Proc. Natl. Acad. Sci. USA 95:6930.
- Dinarello, C.A. (2005) Immunity 23:461.
- Chackerian, A.A. et al. (2007) J. Immunol. 179:2551.
Product Datasheets
Citations for Human IL-33 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
2
Citations: Showing 1 - 2
Filter your results:
Filter by:
-
Is There Any Difference in the In Situ Immune Response in Active Localized Cutaneous Leishmaniasis That Respond Well or Poorly to Meglumine Antimoniate Treatment or Spontaneously Heal?
Authors: Leite-Silva, J;Oliveira-Ribeiro, C;Morgado, FN;Pimentel, MIF;Lyra, MR;Fagundes, A;Miranda, LFC;Valete-Rosalino, CM;Schubach, AO;Conceição-Silva, F;
Microorganisms
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4.
Authors: Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, McKenzie AN, Teixeira MM, Liew FY, Xu D
J. Immunol., 2008-10-01;181(7):4780-90.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human IL-33 Antibody
Average Rating: 4 (Based on 1 Review)
Have you used Human IL-33 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
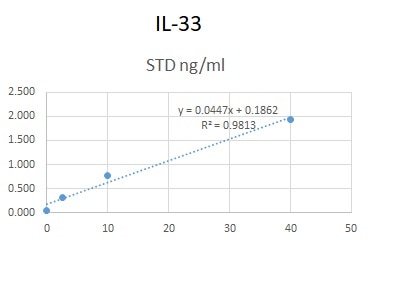
We used this antibody in an in-house ELISA along with pAb (AF3625) and protein (3625-IL-010) to quantify IL-33 in human serum and plasma. This combination could not detect IL-33 in our samples but generated a good standard curve.