Human SIGIRR Antibody Summary
Met1-His118
Accession # Q6IA17
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
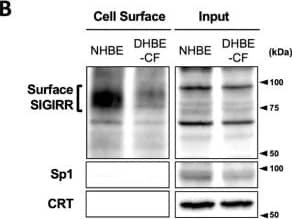
Detection of SIGIRR by Western Blot The expression of the cell surface of the SIGIRR is downregulated in CF cells. (A,B) Cell surface expression of the SIGIRR in (A) C38 and IB3-1, (B) NHBE and DHBE-CF (non-CF vs. CF) airway epithelial cells were assessed by biotinylation assay, followed by immunoblotting using anti-SIGIRR, calreticulin (CRT) and Sp1 antibodies. Protein bands in (A) were scanned, and relative band intensities were normalized to the beta -actin band. The graphs represent the average relative band intensity with S.D. from four independent experiments. * p < 0.05; Student’s t-test (n = 3). (C) Flow cytometry for the SIGIRR was performed using C38 and IB3-1 cells. Surface expressions of the SIGIRR were indicated by a fluorescence shift compared to the isotype control antibody (dotted line). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.
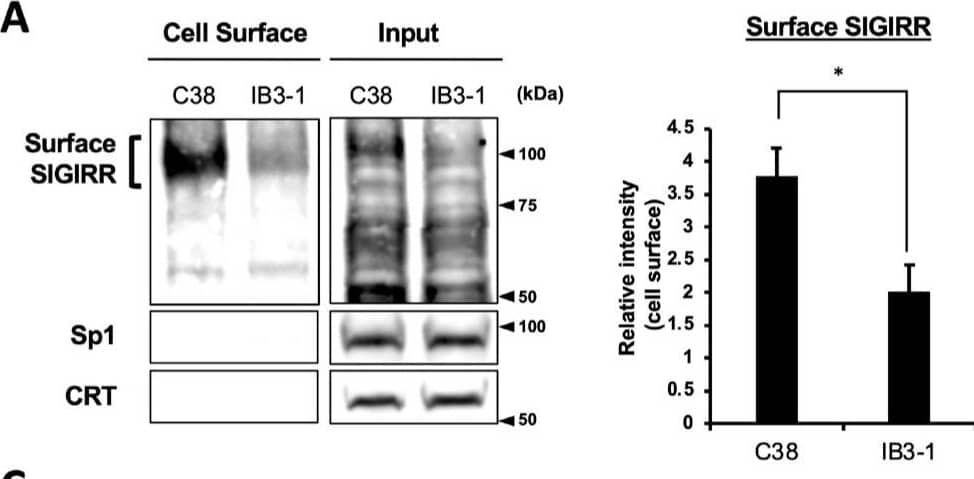 View Larger
View Larger
Detection of SIGIRR by Western Blot The expression of the cell surface of the SIGIRR is downregulated in CF cells. (A,B) Cell surface expression of the SIGIRR in (A) C38 and IB3-1, (B) NHBE and DHBE-CF (non-CF vs. CF) airway epithelial cells were assessed by biotinylation assay, followed by immunoblotting using anti-SIGIRR, calreticulin (CRT) and Sp1 antibodies. Protein bands in (A) were scanned, and relative band intensities were normalized to the beta -actin band. The graphs represent the average relative band intensity with S.D. from four independent experiments. * p < 0.05; Student’s t-test (n = 3). (C) Flow cytometry for the SIGIRR was performed using C38 and IB3-1 cells. Surface expressions of the SIGIRR were indicated by a fluorescence shift compared to the isotype control antibody (dotted line). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
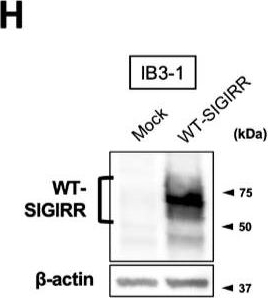
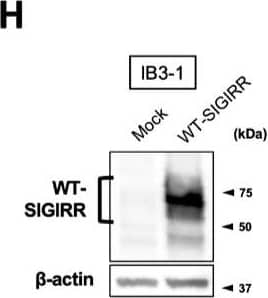
Detection of SIGIRR by Western Blot Suppression of IL-8 production by IL-37b is dependent on the cell surface-expressed WT-SIGIRR, leading to the attenuation of JNK phosphorylation. (A,B) C38 and IB3-1 cells were stimulated with 1 μg/mL flagellin or 20 μg/mL poly(I:C) 2 h after treatment with the indicated concentrations of a precursor or Val46 IL-37b. After 24 h, released IL-8 in the condition media was measured by ELISA. (C) NHBE and DHBE-CF cells were stimulated with 0.5 μg/mL poly(I:C) 2 h after treatment with 10 ng/mL of a precursor or Val46 IL-37b. After 24 h, released IL-8 in the condition media was measured by ELISA. (D) C38 and IB3-1 cells and (E) NHBE and DHBE-CF cells were stimulated with poly(I:C) (D, 20 μg/mL; E, 1 μg/mL) 2 h after treatment with a 10 ng/mL precursor of IL-37b. After the indicated period of the poly(I:C) treatment, the total cell lysates were subjected to immunoblotting using antibodies against the phospho-active form of MAPKs, IRF3, and I kappa B alpha. (F,G) pcDNA or delta 8-SIGIRR-transfected C38 cells and (H and I) pcDNA or the WT-SIGIRR-transfected IB3-1 cells were stimulated with 20 μg/mL poly(I:C) 2 h after treatment with the indicated concentrations of the precursor of IL-37b. After 24 h, released IL-8 in the condition media was measured by ELISA. Exogenously expressed delta 8 and the WT-SIGIRR were assessed by immunoblotting with the anti-SIGIRR. For the immunoblotting experiments (D–F,H), beta -actin was used as a loading control. (A–C,G,I) Results represent the mean ± S.D. * p < 0.05, ** p < 0.01, *** p < 0.001 versus poly(I:C)-treated cells; ANOVA with Dunnett’s test (n = 3). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
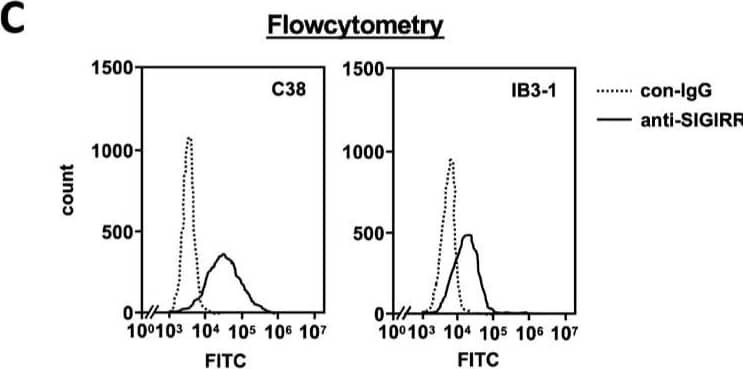
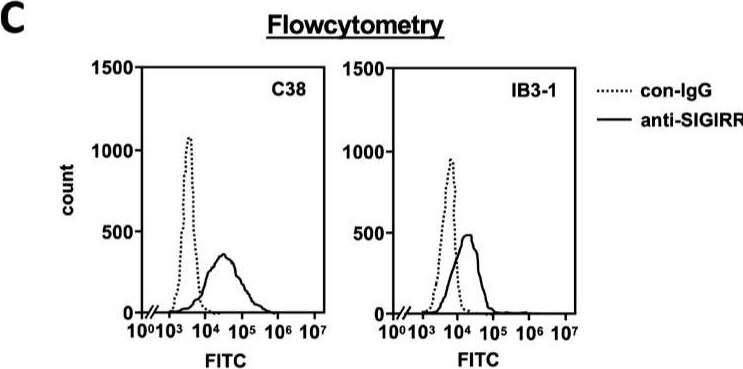
Detection of SIGIRR by Flow Cytometry The expression of the cell surface of the SIGIRR is downregulated in CF cells. (A,B) Cell surface expression of the SIGIRR in (A) C38 and IB3-1, (B) NHBE and DHBE-CF (non-CF vs. CF) airway epithelial cells were assessed by biotinylation assay, followed by immunoblotting using anti-SIGIRR, calreticulin (CRT) and Sp1 antibodies. Protein bands in (A) were scanned, and relative band intensities were normalized to the beta -actin band. The graphs represent the average relative band intensity with S.D. from four independent experiments. * p < 0.05; Student’s t-test (n = 3). (C) Flow cytometry for the SIGIRR was performed using C38 and IB3-1 cells. Surface expressions of the SIGIRR were indicated by a fluorescence shift compared to the isotype control antibody (dotted line). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of SIGIRR by Western Blot Suppression of IL-8 production by IL-37b is dependent on the cell surface-expressed WT-SIGIRR, leading to the attenuation of JNK phosphorylation. (A,B) C38 and IB3-1 cells were stimulated with 1 μg/mL flagellin or 20 μg/mL poly(I:C) 2 h after treatment with the indicated concentrations of a precursor or Val46 IL-37b. After 24 h, released IL-8 in the condition media was measured by ELISA. (C) NHBE and DHBE-CF cells were stimulated with 0.5 μg/mL poly(I:C) 2 h after treatment with 10 ng/mL of a precursor or Val46 IL-37b. After 24 h, released IL-8 in the condition media was measured by ELISA. (D) C38 and IB3-1 cells and (E) NHBE and DHBE-CF cells were stimulated with poly(I:C) (D, 20 μg/mL; E, 1 μg/mL) 2 h after treatment with a 10 ng/mL precursor of IL-37b. After the indicated period of the poly(I:C) treatment, the total cell lysates were subjected to immunoblotting using antibodies against the phospho-active form of MAPKs, IRF3, and I kappa B alpha. (F,G) pcDNA or delta 8-SIGIRR-transfected C38 cells and (H and I) pcDNA or the WT-SIGIRR-transfected IB3-1 cells were stimulated with 20 μg/mL poly(I:C) 2 h after treatment with the indicated concentrations of the precursor of IL-37b. After 24 h, released IL-8 in the condition media was measured by ELISA. Exogenously expressed delta 8 and the WT-SIGIRR were assessed by immunoblotting with the anti-SIGIRR. For the immunoblotting experiments (D–F,H), beta -actin was used as a loading control. (A–C,G,I) Results represent the mean ± S.D. * p < 0.05, ** p < 0.01, *** p < 0.001 versus poly(I:C)-treated cells; ANOVA with Dunnett’s test (n = 3). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of SIGIRR by Flow Cytometry The expression of the cell surface of the SIGIRR is downregulated in CF cells. (A,B) Cell surface expression of the SIGIRR in (A) C38 and IB3-1, (B) NHBE and DHBE-CF (non-CF vs. CF) airway epithelial cells were assessed by biotinylation assay, followed by immunoblotting using anti-SIGIRR, calreticulin (CRT) and Sp1 antibodies. Protein bands in (A) were scanned, and relative band intensities were normalized to the beta -actin band. The graphs represent the average relative band intensity with S.D. from four independent experiments. * p < 0.05; Student’s t-test (n = 3). (C) Flow cytometry for the SIGIRR was performed using C38 and IB3-1 cells. Surface expressions of the SIGIRR were indicated by a fluorescence shift compared to the isotype control antibody (dotted line). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
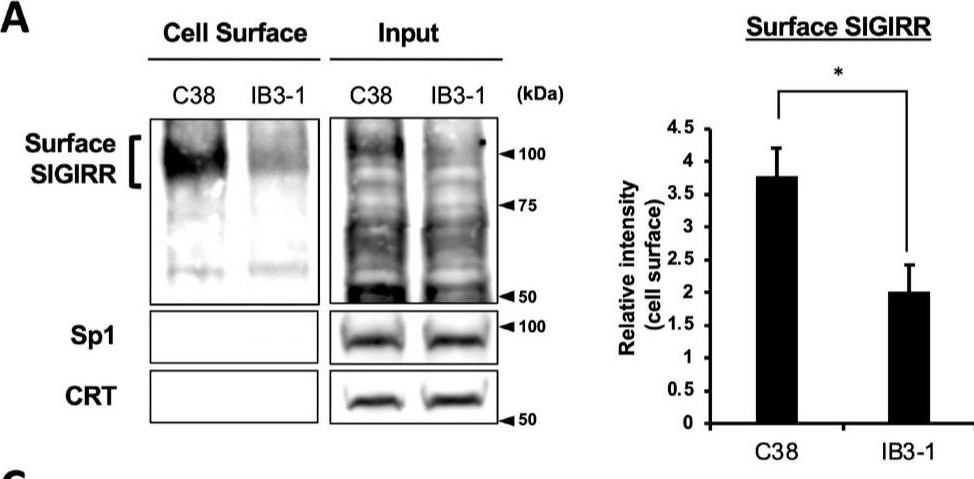
Detection of SIGIRR by Western Blot The expression of the cell surface of the SIGIRR is downregulated in CF cells. (A,B) Cell surface expression of the SIGIRR in (A) C38 and IB3-1, (B) NHBE and DHBE-CF (non-CF vs. CF) airway epithelial cells were assessed by biotinylation assay, followed by immunoblotting using anti-SIGIRR, calreticulin (CRT) and Sp1 antibodies. Protein bands in (A) were scanned, and relative band intensities were normalized to the beta -actin band. The graphs represent the average relative band intensity with S.D. from four independent experiments. * p < 0.05; Student’s t-test (n = 3). (C) Flow cytometry for the SIGIRR was performed using C38 and IB3-1 cells. Surface expressions of the SIGIRR were indicated by a fluorescence shift compared to the isotype control antibody (dotted line). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of SIGIRR by Western Blot The expression of the cell surface of the SIGIRR is downregulated in CF cells. (A,B) Cell surface expression of the SIGIRR in (A) C38 and IB3-1, (B) NHBE and DHBE-CF (non-CF vs. CF) airway epithelial cells were assessed by biotinylation assay, followed by immunoblotting using anti-SIGIRR, calreticulin (CRT) and Sp1 antibodies. Protein bands in (A) were scanned, and relative band intensities were normalized to the beta -actin band. The graphs represent the average relative band intensity with S.D. from four independent experiments. * p < 0.05; Student’s t-test (n = 3). (C) Flow cytometry for the SIGIRR was performed using C38 and IB3-1 cells. Surface expressions of the SIGIRR were indicated by a fluorescence shift compared to the isotype control antibody (dotted line). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35887095), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: SIGIRR
The Interleukin 1 receptor family (IL-1 R) comprises at least eleven members including IL-1 RI (IL-1 R1), IL-1 RII (IL-1 R2), IL-1 RAcP (IL‑1 R3), ST2 (T1/IL-1 R4), IL-18 Ra (IL-1 Rrp/IL-1 R5), IL-1 Rrp2 (IL-1 RL2/IL-1 R6), IL-18 Rb (AcPL/IL-1 R7), IL-1RAPL‑1 (TIGIRR‑2/IL1RAPL1), and TIGIRR-1 (IL-1 R9) (1). All family members possess three immunoglobulin (Ig)-like domains in their extracellular region. Most members have an intracellular TIR (Toll-like receptor/IL-1 receptor signaling) domain that is also conserved in the Toll-like receptor family. Five of the IL-1 R family members (1, 2, 4, 5, and 6) are clustered and localized to chromosome 2. SIGIRR (single Ig domain containing IL-1 receptor-related molecule) is a subtype of the IL-1 R family that differs from the other nine members by having only one Ig domain in its extracellular region. The sequence of human SIGIRR predicts a 410 amino acid (aa) residue transmembrane glycoprotein that lacks signal peptide and contains a 118 aa single Ig extracellular domain, a transmembrane region and a 268 aa cytoplasmic tail with a TIR domain. The cytoplasmic tail of SIGIRR contains a C-terminal extention beyond the TIR domain which is also found in IL1RAPL1, IL-1 R9, and Toll-like receptor family members but absent in other IL-1 receptor family members. SIGIRR is widely expressed and is present in all cells and tissues examined. Mouse and human SIGIRR share 82% amino acid sequence identity. The ligand and signaling mechanism for SIGIRR has not been identified.
Product Datasheets
Citations for Human SIGIRR Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
4
Citations: Showing 1 - 4
Filter your results:
Filter by:
-
A Splice Switch in SIGIRR Causes a Defect of IL-37-Dependent Anti-Inflammatory Activity in Cystic Fibrosis Airway Epithelial Cells
Authors: K Ueno-Shuto, S Kamei, M Hayashi, A Fukuyama, Y Uchida, N Tokutomi, MA Suico, H Kai, T Shuto
International Journal of Molecular Sciences, 2022-07-13;23(14):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, Western Blot -
Staphylococcus aureus intra-mammary infection affects the expression pattern of IL-R8 in goat
Authors: J Filipe, V Bronzo, G Curone, B Castiglion, D Vigo, B Smith, V Herrera, P Roccabianc, P Moroni, F Riva
Comp. Immunol. Microbiol. Infect. Dis., 2019-07-30;66(0):101339.
Species: Goat
Sample Types: Whole Tissue
Applications: IHC-P -
Lipopolysaccharide decreases single immunoglobulin interleukin-1 receptor-related molecule (SIGIRR) expression by suppressing specificity protein 1 (Sp1) via the Toll-like receptor 4 (TLR4)-p38 pathway in monocytes and neutrophils.
Authors: Ueno-Shuto K, Kato K, Tasaki Y, Sato M, Sato K, Uchida Y, Sakai H, Ono T, Suico M, Mitsutake K, Tokutomi N, Kai H, Shuto T
J Biol Chem, 2014-05-12;289(26):18097-109.
Species: Human, Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
The single IgG IL-1-related receptor controls TLR responses in differentiated human intestinal epithelial cells.
Authors: Khan MA, Steiner TS, Sham HP, Bergstrom KS, Huang JT, Assi K, Salh B, Tai IT, Li X, Vallance BA
J. Immunol., 2010-02-03;184(5):2305-13.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human SIGIRR Antibody
There are currently no reviews for this product. Be the first to review Human SIGIRR Antibody and earn rewards!
Have you used Human SIGIRR Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image

