Iressa
Tocris Bioscience | Catalog # 3000

Key Product Details
Description
Alternative Names
Product Description
Iressa is an orally active, selective inhibitor of EGFR tyrosine kinase (IC50 = 23 - 79 nM). Shows minimal activity against ErbB2, KDR, c-flt, PKC, MEK and ERK-2. Blocks EGFR autophosphorylation and inhibits tumor growth in mice bearing a range of human xenografts. Also inhibits growth of brain metastases in mouse model of non-small cell lung cancer.
View information regarding the usage of Iressa for non-clinical studies.
Licensing Information
Sold for research purposes only under agreement from AstraZeneca
Product Specifications for Iressa
Molecular Weight
Formula
Storage
Purity
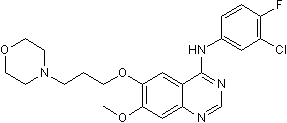
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 44.69 | 100 | |
| Ethanol | 4.47 | 10 |
Preparing Stock Solutions for Iressa
The following data is based on the product molecular weight 446.90.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 2.24 mL | 11.19 mL | 22.38 mL |
| 5 mM | 0.45 mL | 2.24 mL | 4.48 mL |
| 10 mM | 0.22 mL | 1.12 mL | 2.24 mL |
| 50 mM | 0.04 mL | 0.22 mL | 0.45 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 338 publications citing the usage of this product.
- Tan Tyrosine kinase inhibitors show different anti-brain metastases efficacy in NSCLC: A direct comparative analysis of icotinib, gefitinib, and erlo. in a nude mouse model. Oncotarget. 2017 PMID: 29228726
- Baselga ZD1839 ('Iressa'), as an anticancer agent. Drugs 2000 PMID: 11129170
- McKillop Tumor penetration of gefi. (Iressa), an epidermal growth factor receptor tyrosine kinase inhibitor. Mol.Cancer Ther. 2005 PMID: 15827338
- Ciardiello Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin.Cancer Res. 2000 PMID: 10815932
Product Documents for Iressa
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Iressa
For research use only
Related Research Areas
Citations for Iressa
Customer Reviews for Iressa
There are currently no reviews for this product. Be the first to review Iressa and earn rewards!
Have you used Iressa?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
