R&D Systems Antibody Validation

Antibody Validation & Quality Control For Reproducible Results
Life science research and diagnostic development heavily rely on antibodies as targeting reagents, underscoring their importance as fundamental biological tools.
Nevertheless, it’s all too common that commercial antibody reagents do not dependably function as expected. Inconsistent and unreliable antibody performance is a major contributor to poor experimental reproducibility. One estimate suggested that ~50% of antibody products either do not bind their target effectively, struggle across experimental applications, or suffer from off-target binding.
Life science and diagnostic teams need an antibody supplier they can fully trust and depend on to provide reproducible antibody products for all their targets every time they order.
As a recognized leader in the development and validation of antibodies, R&D Systems takes the antibody reproducibility challenge seriously. Through decades of antibody design, development, and manufacturing experience, R&D Systems has continued to enhance and standardize antibody quality control analysis.
In combination with our well-vetted development and manufacturing processes, we apply a stringent application-focused antibody validation workflow to deliver exceptional quality and reliable performance across our extensive antibody catalog–-providing proven reagents designed for diverse targets to researchers the world over.
R&D Systems Antibodies: Exceptional Quality, Proven Applications
Every R&D Systems antibody product, including both off-the-shelf and custom options, goes through our proprietary development processes and validation workflow. To provide our customers with antibodies with reproducible application performance, R&D Systems applies three integral technical approaches:
- Rational Immunogen Design
- Diversified Immunization Strategies
- Enhanced Validation & Verification
1. Rational Immunogen Design
Creating an antibody that provides reproducible, optimal performance starts with thoughtful immunogen selection. At R&D Systems, we design immunogens that lead to better antibodies.
Our in-house protein experts direct the immunogen design process, and oftentimes, they opt for full-length proteins expressed in mammalian cells. Through this approach, we can ensure immunogens are properly folded, bioactive, and appropriately glycosylated so that they behave just as they would in native conditions. Though more difficult to accomplish compared to peptides or protein fragment immunogen approaches, our process yields antibodies that are purposely suited for customer assays.
The resulting antibodies show high selectivity, affinity, and biological relevance. While full-length protein immunogens often provide ideal performance, there are also many cases where a specific antibody target or application necessitates the design of peptide and whole-cell immunogens. The latter is particularly valuable for binding membrane-bound receptors, like GPCR targeting antibodies
2. Diversified Immunization Strategies
With an immunogen selected, our scientists then work to understand which immunization strategy best fits the target antibody. Through years of development, we have built a number of different immunization protocols. Since the antibodies they produce are not necessarily created equal, diverse options help hone in on the best performers.
R&D Systems can also immunize different species, including mouse, rabbit, and goat hosts. We can even immunize multiple hosts with the same immunogen and study the resulting antibodies to collect the best option for specific applications.
3. Enhanced Validation & Verification
R&D Systems performs extensive in-house validation with a keen focus on customer application to provide the highest standards of antibody reproducibility.
Application-Led Antibody Validation
Our validation cycle explores antibody performance in relevant applications like Western blots, flow cytometry, IHC, and other immunoassays. To investigate specificity, all antibodies are tested for cross-reactivity against closely related molecules, facilitated by our extensive library of in-house developed immunogens. Where applicable, we take validation further to functional assays like blocking, neutralization, and receptor activation studies to confirm biological activity.
R&D Systems runs these application validation studies using lysates from primary cells, cell lines, transfectants, and tissue microarrays commonly used in our customers’ experiments.
R&D Systems also performs platform-specific validation on our in-house technologies, like Lunaphore COMET and Simple Western. For customers who use or are considering our instrument platforms, this creates an added layer of confidence that the antibodies will perform as expected.
To assess specificity more deeply, R&D Systems also runs cell-based assays to verify that binding only occurs in the presence of the target, helping to avoid off-target or nonspecific binding.
As most antibody validation experts recommend, this begins with cell studies comparing wild-type cells and isogenic knockout lines lacking the target. In this context, only the wild-type cells show antibody binding. Similarly, our antibody quality control team also uses biological or chemical stimulation assays to verify that target binding only occurs in the appropriate biological context. To illustrate this, using our Hif-1 alpha antibody as an example, we check that binding only occurs in hypoxic conditions since it is only expressed then.
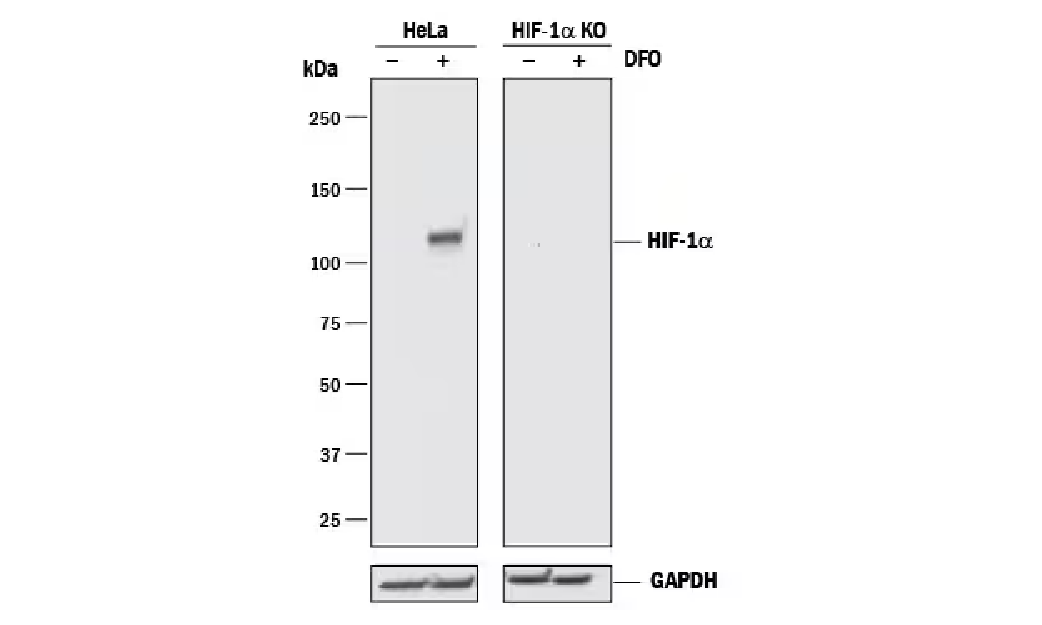
Western Blot Shows Human HIF-1 alpha/HIF1A alpha/HIF1A Specificity. Western blot shows lysates of Wild-type and and HIF-1 alpha/HIF1A knockout cell line (KO) untreated (-) or treated (+) with 1 mM DFO overnight. PVDF membrane was probed with 1 µg/mL of Mouse Anti-Human/Mouse/Rat HIF-1 alpha/HIF1A Monoclonal Antibody (Catalog # MAB1536) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). GAPDH (Catalog # MAB5718) is shown as a loading control.
Ongoing Antibody Quality Control and Lot Confirmation
To ensure sustained performance, R&D Systems runs antibody validation assays for every subsequent lot, testing against a validation standard. By retesting lots, we can help maintain reproducible results in customer experiments. If lots do not perform consistently due to hybridoma drift or any other change, we discontinue the product immediately and develop a comparable alternative.
External Antibody Characterization Partnerships
Since antibody reproducibility affects everyone, antibody suppliers and researchers need to work together to raise our standards. For our part, R&D Systems continues to work closely with external partners to help lead initiatives that strengthen antibody validation.

YCharOS is a public-interest, open-science company with the mission of characterizing commercially available reagent antibodies for every human protein.
Check out this Nature Protocols paper on YCharOS, which shares a consensus-built platform for antibody characterization, including input from our expert antibody team.

Human Cell Differentiation Molecules (HCDM) is an organization that runs Human Leucocyte Differentiation Antigens (HLDA) Workshops and names and characterizes CD molecules.
The HLDA has designated a sizable collection of R&D Systems developed antibody clones as definitive CD markers. Browse our HLDA-qualified CD Markers.
Citations and Customer Reviews
Beyond in-house validation, customer experience is a vital feedback loop for our antibody reproducibility efforts. For transparency, we display publication citations and reviews on every product page in our portfolio so that users can see how other customers successfully use our antibodies in their experiments. Look for these key indicators on the product pages to show how customers have used our antibodies.

100% Guarantee & Expert Technical Support
Even once the antibodies leave our sites, R&D Systems continues to stand by our antibody products, offering a 100% performance guarantee. If a specific product fails to perform consistently, we either refund our customers or replace the product at no additional cost.
Should you encounter any problem with our antibodies, our technical support team is ready to help and answer your questions.
