Recombinant Human GDNF GMP Protein, CF Summary
Product Specifications
Gly113 - Ile211 with & without an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
BT-GDNF-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in sterile water. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
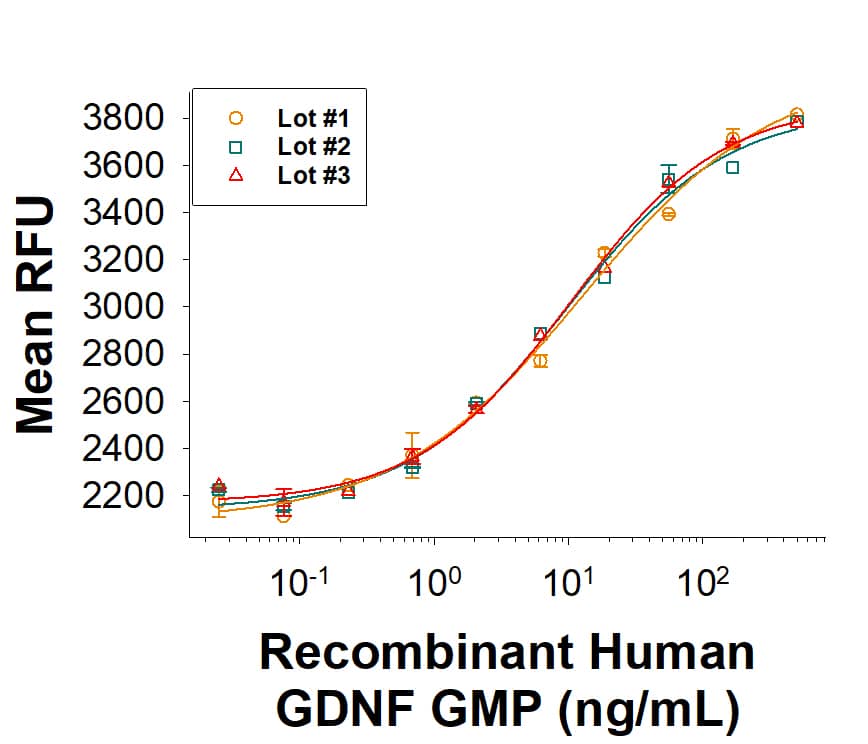
The bioactivity of Recombinant Human GDNF GMP Protein (Catalog # BT-GDNF-GMP) was measured in a cell proliferation assay using the cell proliferation of SH-SY5Y cells. Three independent lots weretested for bioactivity and plotted on the same graph to show lot-to-lot consistency of GMP GDNF protein.
 View Larger
View Larger
Equivalent bioactivity of GMP (Catalog # BT-GDNF-GMP) and Animal-Free (BT-GDNF-AFL) grades of Recombinant HumanGDNF as measured in a cell proliferation assay using the cell proliferation of SH-SY5Y cells (orange and green, respectively).
 View Larger
View Larger
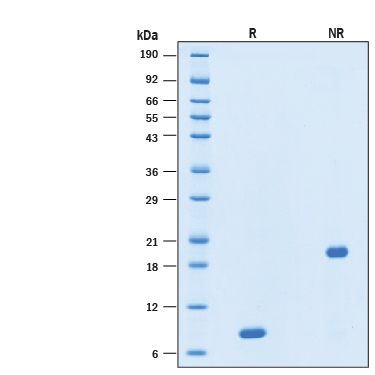
2 μg/lane of Recombinant Human GDNF GMP Protein (Catalog # BT-GDNF-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing a band at 8 kDa under reducing conditions.
Reconstitution Calculator
Background: GDNF
Glial Cell Line-derived Neurotrophic Factor (GDNF) is a neurotrophic factor that has been shown to promote the survival of various neuronal subpopulations in both the central as well as the peripheral nervous systems at different stages of their development. Neuronal subpopulations that have been shown to be affected by GDNF include motoneurons, midbrain dopaminergic neurons, Purkinje cells and sympathetic neurons.
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers.
Product Specific Notices
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human GDNF GMP Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant Human GDNF GMP Protein, CF and earn rewards!
Have you used Recombinant Human GDNF GMP Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image

