Recombinant Mouse Serpin F1/PEDF Protein, CF Summary
- R&D Systems NS0-derived Recombinant Mouse Serpin F1/PEDF Protein (8295-SF)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Product Specifications
Gln20-Thr417, with a C-terminal 6-His tag
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
8295-SF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS and NaCl. |
| Reconstitution | Reconstitute at 250 μg/mL in steril PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
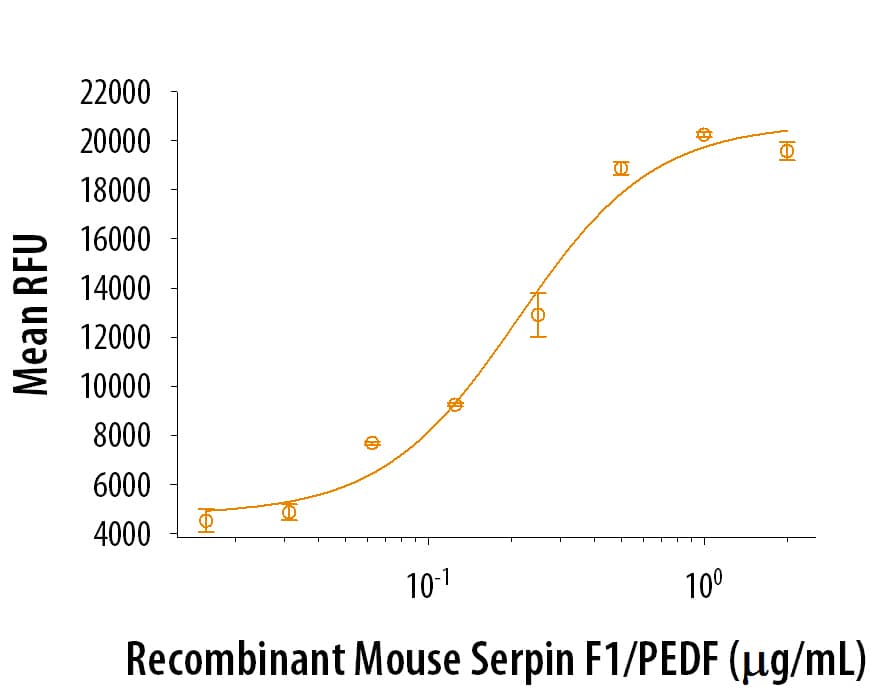 View Larger
View Larger
Recombinant Mouse Serpin F1/PEDF enhances the adhesion of Saos‑2 human osteosarcoma cells to a bovine Collagen I coated plate. The ED50 for this effect is ≤ 2.00 μg/mL.
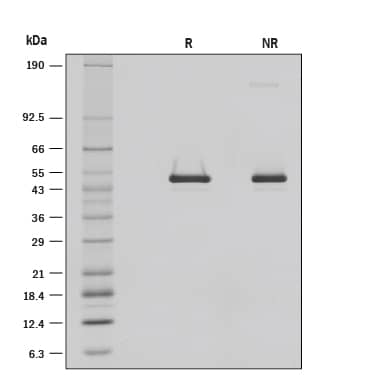 View Larger
View Larger
1 μg/lane of Recombinant Mouse Serpin F1/PEDF was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by silver staining, showing R bands at 48.8, 42.5 kDa and NR bands at 140.8, 47.9, 42.1 kDa.
Reconstitution Calculator
Background: Serpin F1/PEDF
Serpin Peptidase Inhibitor, clade F (Serpin F1), also called Pigment Epithelium-Derived Factor (PEDF), EPC-1, and PIG35, is a member of the Serpin superfamily of serine protease inhibitors (1-5). This superfamily is comprised of two protein groups with dissimilar functions. One group demonstrates protease inhibition while proteins in the other group display no protease inhibition, but rather, perform diverse functions, such as molecular chaperones, circulating transporters, and tumor suppressors (2). Serpin F1 is part of this latter group. It is a 50 kDa, monomeric phosphoglycoprotein that is comprised of three beta -sheets, 8-10 alpha -helices, and a C-terminal reactive center loop (RCL), a structure common to all Serpins (2-5). However, unlike Serpins that exhibit protease inhibiting activity, the RCL of Serpin F1 does not undergo a conformational change, a prerequisite for anti-protease activity (3, 5). Such cleavage does, however, generate a 46 kDa fragment that possesses nonprotease-associated bioactivity (6). Mouse Serpin F1 displays 88% and 93% amino acid sequence identity with the human and rat orthologs, respectively.
Serpin F1 is a multifunctional protein that is synthesized by multiple cell types and is expressed in many tissues including the retinal pigment epithelium, liver, bone, connective, heart, and adipose tissues (1, 3, 5, 7, 8). It has been shown to bind to several different cell surface molecules including the PEDF Receptor, Laminin Receptor, LRP-6, and the F1 ATP Synthase (5, 9-13). It also has binding affinity for several extracellular matrix components, such as Heparin, Heparin Sulphate, Hyaluronan, and Collagens (5, 3). It is believed that the multiple and varied biological activities attributed to Serpin F1 are due to its interactions with these different cell surface molecules. Serpin F1 has been shown to be involved in neurogenesis, neuronal cell survival, angiogenesis, tumorgenesis, stem cell survival and multipotency, and inflammation (2-5, 10, 12-15). In humans, Serpin F1 has been suggested to play a role in choroidal neovascularization, obesity and insulin resistance, cardiovascular disease, osteogenesis imperfecta, and cancer (3-5, 15-19).
- Kozaki, K. et al. (1998) J. Biol. Chem. 273:15125.
- Filleur, S. et al. (2009) J. Cell. Biochem. 106:769.
- Kawaguchi, T. et al. (2010) Curr. Mol. Med. 10:302.
- Chandolu, V. and C.R. Dass (2012) J. Biomed. Biotechnol. 2012:740295.
- Becerra, S.P. and V. Notario (2013) Nat. Rev. Cancer 13:258.
- Wu, Y.Q. and S.P. Becerra (1996) Invest. Ophthalmol. Vis. Sci. 37:1984.
- Singh, V.K. et al. (1998) Mol. Vis. 4:7.
- Tombran-Tink, J. and C.J. Barnstable (2004) Biochem. Biophys. Res. Commun. 316:573.
- Notari, L. et al. (2006) J. Biol. Chem. 281:38022.
- Notari, L. et al. (2010) FEBS J. 277:2192.
- Park, K. et al. (2011) Mol. Cell. Biol. 31:3038.
- Bernard, A. et al. (2009) J. Biol. Chem. 284:10480.
- Matsui, T. et al. (2014) Biochem. Biophys. Res. Commun. 443:847.
- Elahy, M. et al. (2012) J. Biomed. Biotechol. 2012:239091.
- Chavan, S.S. et al. (2012) Mol. Med. 18:1161.
- Wang, P. et al. (2008) Eur. J. Endocrinol. 159:713.
- Homan, E.P. et al. (2011) J. Bone Miner. Res. 26:2798.
- Taube, A. et al. (2012) Am. J. Physiol. Heart Circ. Physiol. 302:H2148.
- Venturi, G. et al. (2012) J. Bone Miner. Res. 27:723.
Citations for Recombinant Mouse Serpin F1/PEDF Protein, CF
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
2
Citations: Showing 1 - 2
Filter your results:
Filter by:
-
Polarized RPE Secretome Preserves Photoreceptors in Retinal Dystrophic RCS Rats
Authors: Ahluwalia, K;Martinez-Camarillo, JC;Thomas, BB;Naik, A;Gonzalez-Calle, A;Pollalis, D;Lebkowski, J;Lee, SY;Mitra, D;Louie, SG;Humayun, MS;
Cells
Species: Rat
Sample Types: In Vivo
Applications: In Vivo -
Pigment Epithelium-Derived Factor (PEDF) Receptors Are Involved in Survival of Retinal Neurons
Authors: S Bürger, J Meng, A Zwanzig, M Beck, M Pankonin, P Wiedemann, W Eichler, JD Unterlauft
International Journal of Molecular Sciences, 2020-12-31;22(1):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Mouse Serpin F1/PEDF Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant Mouse Serpin F1/PEDF Protein, CF and earn rewards!
Have you used Recombinant Mouse Serpin F1/PEDF Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
