Recombinant Mouse TRANCE/RANK L/TNFSF11 (E. coli-expressed)
Recombinant Mouse TRANCE/RANK L/TNFSF11 (E. coli-expressed) Summary
- R&D Systems E. coli-derived Recombinant Mouse TRANCE/RANK L/TNFSF11 (E. coli-expressed) (462-TEC)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Product Specifications
Lys158-Asp316, with an N-terminal Met
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
462-TEC
| Formulation | Lyophilized from a 0.2 μm filtered solution in NaH2PO4, NaCl and EDTA with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
462-TEC/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in NaH2PO4, NaCl and EDTA. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
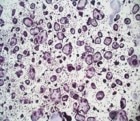
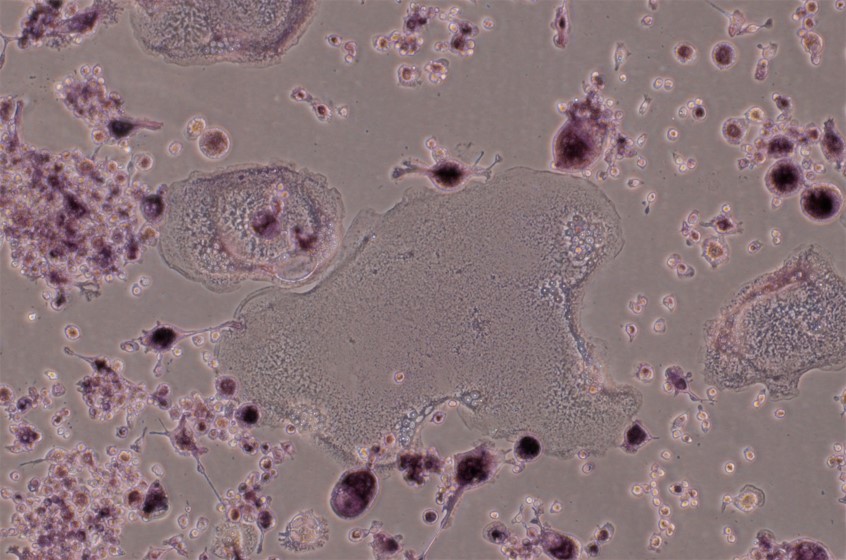
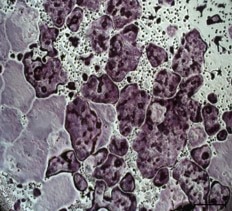
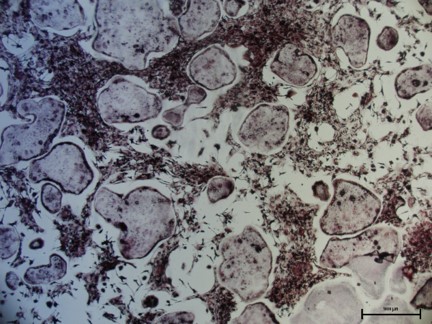
Recombinant Mouse TRANCE/TNFSF11/RANK L (Catalog # 462-TEC) induces osteoclast differentiation of the RAW 264.7 mouse monocyte/macrophage cell line. The ED50 for this effect is 0.5-2 ng/mL.
 View Larger
View Larger
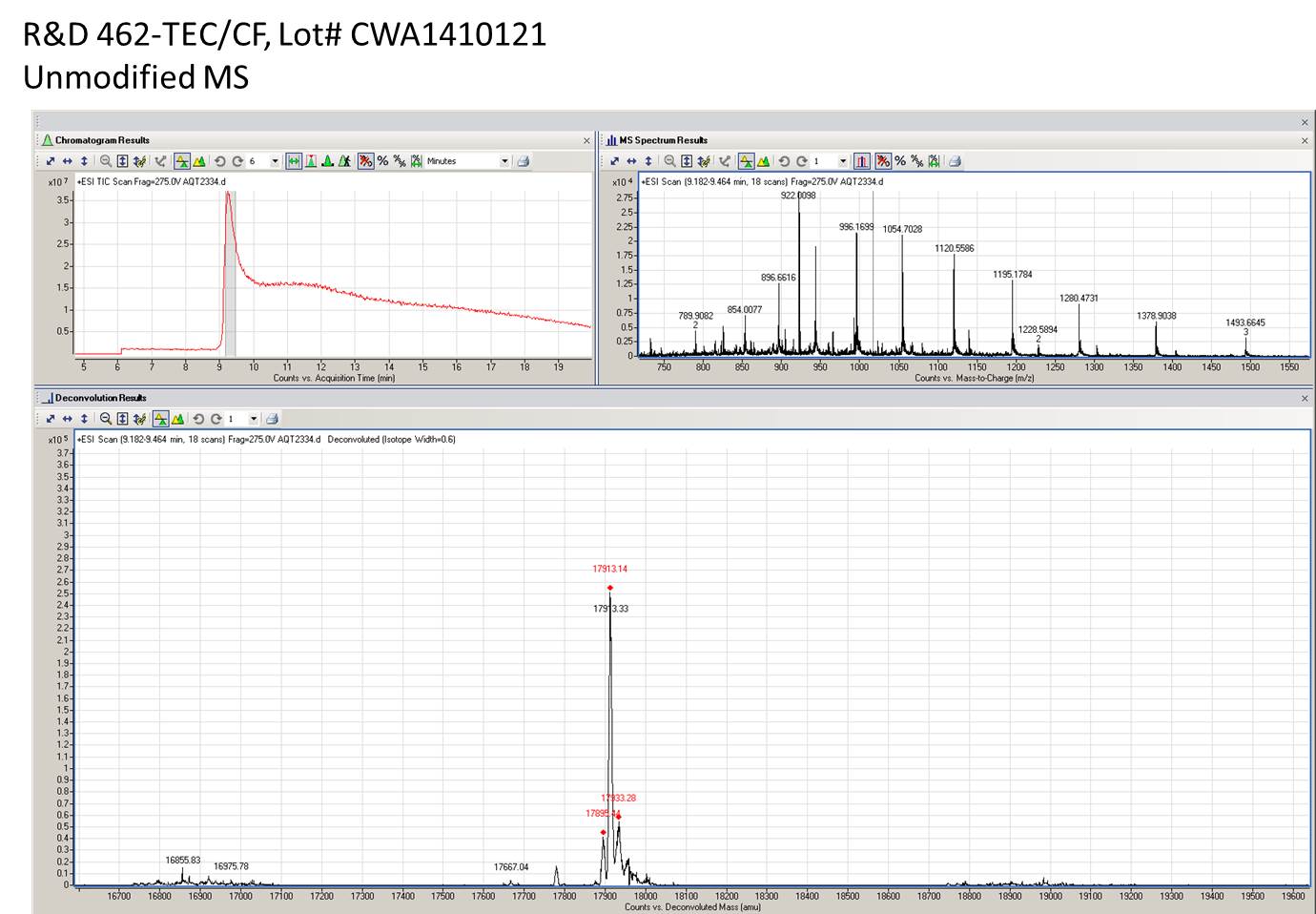
1 μg/lane of Recombinant Mouse TRANCE/TNFSF11/RANK L was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 19 kDa.
Reconstitution Calculator
Background: TRANCE/TNFSF11/RANK L
TRANCE (receptor activator of NF-kappa B ligand [RANK L], also called TNF-related activation-induced cytokine [TRANCE], osteoprotegrin ligand [OPGL], and osteoclast differentiation factor [ODF]), is a member of the tumor necrosis factor (TNF) family. TRANCE was originally identified as an immediate early gene up-regulated by T cell receptor stimulation. The mouse TRANCE cDNA encodes a type II transmembrane protein of 316 amino acids with a predicted cytoplasmic domain of 48 amino acids and an extracellular domain of 247 amino acids. The extracellular domain contains two potential N-linked glycosylation sites. Mouse and human TRANCE share 85% amino acid identity. TRANCE is primarily expressed in T cells and T cell rich organs, such as thymus and lymph nodes. The multi-functions of TRANCE include induction of activation of the c-jun N-terminal kinase, enhancement of T cell growth and dendritic cell function, induction of osteoclastogenesis, and lymph node organogenesis. RANK is the cell surface signaling receptor of TRANCE. RANK has been shown to undergo receptor clustering during signal transduction. Osteoprotegrin, a soluble member of the TNF receptor family which binds TRANCE, is a naturally occurring decoy receptor that counterbalances the effects of TRANCE.
- Wong, B.R. et al. (1997) J. Biol. Chem. 272:25190.
- Anderson, D.M. et al. (1997) Nature 390:175.
- Nakagawa, N. et al. (1998) Biochem. Biophys. Res. Commun. 245:382.
- Kong, Y-Y. et al. (1999) Nature 397:315.
Citations for Recombinant Mouse TRANCE/RANK L/TNFSF11 (E. coli-expressed)
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
106
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
A novel HDAC1-specific inhibitor prevents estrogen deficiency-induced osteoporosis in mice by inhibiting osteoclast function
Authors: Yuan, H;Bao, Y;Li, L;Luan, Y;Li, F;
Archives of gerontology and geriatrics
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Receptor activator of nuclear factor-kappa B ligand-derived microglia healing peptide 1-AcN inhibits osteoarthritis progression in mice
Authors: Fukuda, Y;Shimamura, M;Etani, Y;Noguchi, T;Kurihara, T;Goshima, A;Miura, T;Hirao, M;Ochiai, N;Ju, N;Sugimoto, A;Kanamoto, T;Nakata, K;Okada, S;Ebina, K;
Arthritis research & therapy
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Integrating network pharmacology, IPA, and molecular docking to reveal the anti-osteoporosis effects of EA and EB via the FAK pathway
Authors: Wei, Z;You, F;Li, H;Wu, S;Tang, F;Wan, X;Dong, H;Huang, W;Gao, S;Cai, B;Chen, X;Dong, X;
Frontiers in pharmacology
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Injectable magnesium-bisphosphonate MOF-based bone adhesive prevents excessive fibrosis for osteoporotic fracture repair
Authors: Xiao, T;Gong, Z;Duan, D;Yu, H;Liu, S;Jiang, Y;Xing, X;Wu, Z;Wang, L;Yang, XB;Tronci, G;Ning, C;Tan, G;Zhou, L;
Nature communications
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Binary curcuminoid complex CRE-Bin inhibits osteoclast differentiation by suppressing the canonical NF-?B signaling pathway and modulating microRNA-223 expression
Authors: Jantarawong, S;Khimmaktong, W;Swangphon, P;Lauterbach, N;Nanakorn, N;Panichayupakaranant, P;Pengjam, Y;
Scientific reports
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Engineered Spirulina platensis for treating rheumatoid arthritis and restoring bone homeostasis
Authors: Yang, X;Rong, K;Fu, S;Yang, Y;Liu, S;Zhang, C;Xu, K;Zhang, K;Zhu, Y;Hao, Y;Zhao, J;Fu, J;
Nature communications
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
LY6E as a new prognostic biomarker of multiple myeloma-related bone disease
Authors: Shi, M;Li, J;Wang, J;Yao, Y;Shen, X;Xia, Y;Xu, J;
Scientific reports
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Deletion of kinin receptor B2 enhances orthodontic tooth movement and alveolar bone remodeling
Authors: Figueiredo, NC;Piacsek, M;Montalvany-Antonucci, CC;Santos, MS;Amaral, FA;Teixeira, MM;Silva, TA;Macari, S;Pandruvada, S;Andrade, I;
PloS one
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Cystatin M/E Ameliorates Multiple Myeloma-Induced Hyper Osteolytic Bone Resorption
Authors: Gai, D;Caviness, PC;Lazarenko, OP;Chen, JF;Randolph, CE;Zhang, Z;Cheng, Y;Sun, F;Xu, H;Blackburn, ML;Tricot, G;Shaughnessy, JD;Chen, JR;Zhan, F;
Cancers
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
GATA3-Driven ceRNA Network in Lung Adenocarcinoma Bone Metastasis Progression and Therapeutic Implications
Authors: Liu, Y;Shen, S;Wang, X;Chen, H;Ren, W;Wei, H;Li, K;Li, L;
Cancers
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Targeting TRPC channels for control of arthritis-induced bone erosion
Authors: Ray, S;McCall, JL;Tian, JB;Jeon, J;Douglas, A;Tyler, K;Liu, S;Berry, K;Nicewarner, B;Hall, C;Groschner, K;Bacsa, B;Geldenhuys, W;Zhu, MX;Blair, HC;Barnett, JB;Soboloff, J;
Science advances
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Exploring the In Vitro Effects of Zingerone on Differentiation and Signalling Pathways in Bone Cell Lines
Authors: De Vos, B;Kasonga, AE;Joubert, AM;Nyakudya, TT;
Metabolites
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
NDR2 is critical for the osteoclastogenesis by regulating ULK1-mediated mitophagy
Authors: Kong, X;Shan, Z;Zhao, Y;Tao, S;Chen, J;Ji, Z;Jin, J;Liu, J;Lin, W;Wang, X;Wang, J;Zhao, F;Huang, B;Chen, J;
JCI insight
Species: Mouse
Sample Types:
Applications: Bioassay -
Orthosilicic acid inhibits human osteoclast differentiation and bone resorption
Authors: Magnusson, C;Ransjö, M;
PloS one
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Vasorin-deficient mice display disturbed vitamin D and mineral homeostasis in combination with a low bone mass phenotype
Authors: Eijken, M;Krautzberger, AM;Scholze-Wittler, M;Boers-Sijmons, B;Koedam, M;Kosiol, B;Schrewe, H;van Leeuwen, JP;van der Eerden, BC;
Bone reports
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Crebanine mitigates glucocorticoid-induced osteonecrosis of the femoral head by restoring bone remodelling homeostasis via attenuating oxidative stress
Authors: Dong, S;Ge, J;Meng, Q;Yuan, T;Wang, Y;Li, Y;Lu, Q;Song, W;Li, Z;Sun, S;
Journal of cellular and molecular medicine
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Secretory factors released from high dose radiation-activated osteoclasts increase the expression level of pain-associated neuropeptides in sensory neuronal cultures
Authors: Park, SH;Peters, M;Aguayo, C;Farris, MK;Hughes, RT;Moore, J;Munley, MT;Reno, KE;Gardin, J;Cline, JM;Peters, CM;Willey, JS;
Research square
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Prolonged Cadmium Exposure and Osteoclastogenesis: A Mechanistic Mouse and in Vitro Study
Authors: Liu, Z;Wu, J;Dong, Z;Wang, Y;Wang, G;Chen, C;Wang, H;Yang, Y;Sun, Y;Yang, M;Fu, J;Li, J;Zhang, Q;Xu, Y;Pi, J;
Environmental health perspectives
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells, Transfected Whole Cells
Applications: Bioassay -
Biological and Mechanical Performance of Dual-Setting Brushite-Silica Gel Cements
Authors: Steinacker, VC;Renner, T;Holzmeister, I;Gubik, S;Müller-Richter, U;Breitenbücher, N;Fuchs, A;Straub, A;Scheurer, M;Kübler, AC;Gbureck, U;
Journal of functional biomaterials
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Kisspeptin-10 binding to Gpr54 in osteoclasts prevents bone loss by activating Dusp18-mediated dephosphorylation of Src
Authors: Li, Z;Yang, X;Fu, R;Wu, Z;Xu, S;Jiao, J;Qian, M;Zhang, L;Wu, C;Xie, T;Yao, J;Wu, Z;Li, W;Ma, G;You, Y;Chen, Y;Zhang, HK;Cheng, Y;Tang, X;Wu, P;Lian, G;Wei, H;Zhao, J;Xu, J;Ai, L;Siwko, S;Wang, Y;Ding, J;Song, G;Luo, J;Liu, M;Xiao, J;
Nature communications
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Osteoprotective effect by interleukin-4 (IL-4) on lipoprotein-induced periodontitis
Authors: Lima Teixeira, JF;Henning, P;Cintra Magalhães, FA;Coletto-Nunes, G;Floriano-Marcelino, T;Westerlund, A;Movérare-Skrtic, S;Oliveira, GJPL;Lerner, UH;Souza, PPC;
Cytokine
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Echinococcus granulosus promotes bone resorption by increasing osteoclasts differentiation
Authors: Sun, H;Wang, S;Tan, W;Li, Y;Ren, Q;Liu, Y;Huang, Y;Shi, C;Li, J;
Acta tropica
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
CircFam190a: a critical positive regulator of osteoclast differentiation via enhancement of the AKT1/HSP90? complex
Authors: Chen, K;Chen, X;Lang, C;Yuan, X;Huang, J;Li, Z;Xu, M;Wu, K;Zhou, C;Li, Q;Zhu, C;Liu, L;Shang, X;
Experimental & molecular medicine
Species: Mouse
Sample Types: Whole Cells
Applications: Differentiation -
GV1001 Inhibits the Severity of the Ligature-Induced Periodontitis and the Vascular Lipid Deposition Associated with the Periodontitis in Mice
Authors: Kim, SY;Kim, YJ;Kim, S;Momeni, M;Lee, A;Treanor, A;Kim, S;Kim, RH;Park, NH;
International journal of molecular sciences
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The checkpoint inhibitor PD-1H/VISTA controls osteoclast-mediated multiple myeloma bone disease
Authors: Fu, J;Li, S;Ma, H;Yang, J;Pagnotti, GM;Brown, LM;Weiss, SJ;Mapara, MY;Lentzsch, S;
Nature communications
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Schnurri-3 inhibition suppresses bone and joint damage in models of rheumatoid arthritis
Authors: Stavre, Z;Kim, JM;Yang, YS;N�ndel, K;Chaugule, S;Sato, T;Park, KH;Gao, G;Gravallese, EM;Shim, JH;
Proceedings of the National Academy of Sciences of the United States of America
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
GSTP1-mediated S-glutathionylation of Pik3r1 is a redox hub that inhibits osteoclastogenesis through regulating autophagic flux
Authors: X Ji, J Hong, W Yang, M Yao, J Wang, G Jiang, Y Wang, C Li, J Lin, H Mou, C Li, S Li, Y Chen, M Shi, W Wang, F Lu, H Wu, X Zhao, Y Qi, S Yan
Redox Biology, 2023-02-27;61(0):102635.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Npp1 prevents external tooth root resorption by regulation of cervical cementum integrity
Authors: H Choi, L Yang, Y Liu, JK Jeong, ES Cho
Scientific Reports, 2022-12-07;12(1):21158.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Evaluation of culture conditions for osteoclastogenesis in RAW264.7 cells
Authors: Y Cheng, H Liu, J Li, Y Ma, C Song, Y Wang, P Li, Y Chen, Z Zhang
PLoS ONE, 2022-11-17;17(11):e0277871.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
CRELD2 is a novel modulator of calcium release and calcineurin-NFAT signalling during osteoclast differentiation
Authors: A Duxfield, J Munkley, MD Briggs, EP Dennis
Scientific Reports, 2022-08-16;12(1):13884.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
BHLHE40 promotes osteoclastogenesis and abnormal bone resorption via c-Fos/NFATc1
Authors: Y Zhang, M Yang, S Zhang, Z Yang, Y Zhu, Y Wang, Z Chen, X Lv, Z Huang, Y Xie, L Cai
Cell & bioscience, 2022-05-26;12(1):70.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
D-galactose-induced aging aggravates obesity-induced bone dyshomeostasis
Authors: N Imerb, C Thonusin, W Pratchayas, B Arunsak, W Nawara, B Ongnok, R Aeimlapa, N Charoenpha, N Chattipako, SC Chattipako
Scientific Reports, 2022-05-20;12(1):8580.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Carnosol inhibits osteoclastogenesis in�vivo and in�vitro by blocking the RANKL?induced NF?kappaB signaling pathway
Authors: P Cai, S Yan, Y Lu, X Zhou, X Wang, M Wang, Z Yin
Molecular Medicine Reports, 2022-05-20;26(1):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Network Pharmacology Deciphers the Action of Bioactive Polypeptide in Attenuating Inflammatory Osteolysis via the Suppression of Oxidative Stress and Restoration of Bone Remodeling Balance
Authors: Z Cui, C Feng, J Chen, Y Wang, Q Meng, S Zhao, Y Zhang, D Feng, Z Li, S Sun
Oxidative Medicine and Cellular Longevity, 2022-04-14;2022(0):4913534.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TET2 regulates osteoclastogenesis by modulating autophagy in OVX-induced bone loss
Authors: C Yang, H Tao, H Zhang, Y Xia, J Bai, G Ge, W Li, W Zhang, L Xiao, Y Xu, Z Wang, Y Gu, H Yang, Y Liu, D Geng
Autophagy, 2022-03-24;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
P2X7 receptor knockdown suppresses osteoclast differentiation by inhibiting autophagy and Ca2+/calcineurin signaling
Authors: Y Ma, R Di, H Zhao, R Song, H Zou, Z Liu
Molecular Medicine Reports, 2022-03-10;25(5):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Regulation of sclerostin by the SIRT1 stabilization pathway in osteocytes
Authors: JM Kim, YS Yang, J Xie, O Lee, J Kim, J Hong, B Boldyreff, O Filhol, H Chun, MB Greenblatt, G Gao, JH Shim
Cell Death and Differentiation, 2022-02-15;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Inhibitory Effects of Astaxanthin on CML-HSA-Induced Inflammatory and RANKL-Induced Osteoclastogenic Gene Expression in RAW 264.7 Cells
Authors: ANM Mamun-Or-R, TT Lucy, M Yagi, Y Yonei
Biomedicines, 2021-12-27;10(1):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Galectin-3 Contributes to the Inhibitory Effect of lalpha,25-(OH)2D3 on Osteoclastogenesis
Authors: J Gu, X Zhang, C Zhang, Y Li, J Bian, X Liu, Y Yuan, H Zou, X Tong, Z Liu
International Journal of Molecular Sciences, 2021-12-11;22(24):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
STAT3 is critical for skeletal development and bone homeostasis by regulating osteogenesis
Authors: S Zhou, Q Dai, X Huang, A Jin, Y Yang, X Gong, H Xu, X Gao, L Jiang
Nature Communications, 2021-11-25;12(1):6891.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
ULK1 Suppresses Osteoclast Differentiation and Bone Resorption via Inhibiting Syk-JNK through DOK3
Authors: Y Zhang, S Zhang, Y Wang, Z Yang, Z Chen, N Wen, M Yang, Z Huang, Y Xie, L Cai
Oxidative Medicine and Cellular Longevity, 2021-11-15;2021(0):2896674.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
CNC-bZIP protein NFE2L1 regulates osteoclast differentiation in antioxidant-dependent and independent manners
Authors: Z Liu, H Wang, Y Hou, Y Yang, J Jia, J Wu, Z Zuo, T Gao, S Ren, Y Bian, S Liu, J Fu, Y Sun, J Li, M Yamamoto, Q Zhang, Y Xu, J Pi
Redox Biology, 2021-11-06;48(0):102180.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
RSPO3 is important for trabecular bone and fracture risk in mice and humans
Authors: KH Nilsson, P Henning, ME Shahawy, M Nethander, TL Andersen, C Ejersted, J Wu, KL Gustafsson, A Koskela, J Tuukkanen, PPC Souza, J Tuckermann, M Lorentzon, LE Ruud, T Lehtimäki, JH Tobias, S Zhou, UH Lerner, JB Richards, S Movérare-S, C Ohlsson
Nature Communications, 2021-08-13;12(1):4923.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Diterbutyl phthalate attenuates osteoarthritis in ACLT mice via suppressing ERK/c-fos/NFATc1 pathway, and subsequently inhibiting subchondral osteoclast fusion
Authors: C Fang, JW Guo, YJ Wang, XQ Li, H Zhang, J Cui, Y Hu, YY Jing, X Chen, JC Su
Acta pharmacologica Sinica, 2021-08-11;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
STING suppresses bone cancer pain via immune and neuronal modulation
Authors: K Wang, CR Donnelly, C Jiang, Y Liao, X Luo, X Tao, S Bang, A McGinnis, M Lee, MJ Hilton, RR Ji
Nature Communications, 2021-07-27;12(1):4558.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Plastrum testudinis extract suppresses osteoclast differentiation via the NF-&kappaB signaling pathway and ameliorates senile osteoporosis
Authors: H Chen, G Shen, Q Shang, P Zhang, D Yu, X Yu, Z Zhang, W Zhao, Z Wu, F Tang, Liang, X Jiang, H Ren
Journal of ethnopharmacology, 2021-05-08;276(0):114195.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Deficiency of optineurin enhances osteoclast differentiation by attenuating the NRF2-mediated antioxidant response
Authors: P Xue, X Hu, E Chang, L Wang, M Chen, TH Wu, DJ Lee, BL Foster, HC Tseng, CC Ko
Experimental & Molecular Medicine, 2021-04-16;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Identification of Fibroblast Activation Protein as an Osteogenic Suppressor and Anti-osteoporosis Drug Target
Authors: H Wei, Y Xu, Y Wang, L Xu, C Mo, L Li, B Shen, Y Sun, P Cheng, L Yang, Y Pang, A Qin, Y Cao, SJ Morrison, R Yue
Cell Rep, 2020-10-13;33(2):108252.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
PD-1 blockade inhibits osteoclast formation and murine bone cancer pain
Authors: K Wang, Y Gu, Y Liao, S Bang, CR Donnelly, O Chen, X Tao, AJ Mirando, MJ Hilton, RR Ji
J. Clin. Invest., 2020-07-01;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Dendritic cells-derived interferon-lambda1 ameliorated inflammatory bone destruction through inhibiting osteoclastogenesis
Authors: Y Chen, Y Wang, R Tang, J Yang, C Dou, Y Dong, D Sun, C Zhang, L Zhang, Y Tang, Q Dai, F Luo, J Xu, S Dong
Cell Death Dis, 2020-06-02;11(6):414.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A RUNX2 stabilization pathway mediates physiologic and pathologic bone formation
Authors: JM Kim, YS Yang, KH Park, X Ge, R Xu, N Li, M Song, H Chun, S Bok, JF Charles, O Filhol-Coc, B Boldyreff, T Dinter, PB Yu, N Kon, W Gu, T Takarada, MB Greenblatt, JH Shim
Nat Commun, 2020-05-08;11(1):2289.
Species: Mouse
Sample Types: Whole Cell
Applications: Bioassay -
QKI deficiency leads to osteoporosis by promoting RANKL-induced osteoclastogenesis and disrupting bone metabolism
Authors: T Du, Z Yan, S Zhu, G Chen, L Wang, Z Ye, W Wang, Q Zhu, Z Lu, X Cao
Cell Death Dis, 2020-05-07;11(5):330.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Neural regulation of energy and bone homeostasis by the synaptic adhesion molecule Calsyntenin-3
Authors: SJ Kim, YT Jeong, SR Jeong, M Park, HS Go, MY Kim, JK Seong, KW Kim, JT Seo, CH Kim, JH Lee, SJ Moon
Exp. Mol. Med., 2020-05-07;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Opposing roles of hematopoietic-specific small GTPase Rac2 and the guanine nucleotide exchange factor Vav1 in osteoclast differentiation
Authors: IS Kang, JS Jang, C Kim
Sci Rep, 2020-04-27;10(1):7024.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The IAP Antagonist SM-164 Eliminates Triple-Negative Breast Cancer Metastasis to Bone and Lung in Mice
Authors: W Lei, R Duan, J Li, X Liu, A Huston, BF Boyce, Z Yao
Sci Rep, 2020-04-24;10(1):7004.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Effects of N-methyl-D-aspartate receptor antagonist MK-801 (dizocilpine) on bone homeostasis in mice
Authors: S Kiyohara, N Sakai, K Handa, T Yamakawa, K Ishikawa, M Chatani, A Karakawa, Y Azetsu, M Munakata, M Ozeki, T Negishi-Ko, M Takami
J Oral Biosci, 2020-04-11;0(0):.
Species: Mouse
Sample Types: bone marrow cells
Applications: Cell Culture -
SENP3 Suppresses Osteoclastogenesis by De-conjugating SUMO2/3 from IRF8 in Bone Marrow-Derived Monocytes
Authors: Y Zhang, K Yang, J Yang, Y Lao, L Deng, G Deng, J Yi, X Sun, Q Wang
Cell Rep, 2020-02-11;30(6):1951-1963.e4.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Environmental arginine controls multinuclear giant cell metabolism and formation
Authors: JS Brunner, L Vulliard, M Hofmann, M Kieler, A Lercher, A Vogel, M Russier, JB Brüggenthi, M Kerndl, V Saferding, B Niederreit, A Junza, A Frauenstei, C Scholtysek, Y Mikami, K Klavins, G Krönke, A Bergthaler, JJ O'Shea, T Weichhart, F Meissner, JS Smolen, P Cheng, O Yanes, J Menche, PJ Murray, O Sharif, S Blüml, G Schabbauer
Nat Commun, 2020-01-22;11(1):431.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
A Novel Sulforaphane-Regulated Gene Network in Suppression of Breast Cancer-Induced Osteolytic Bone Resorption
Authors: SK Pore, ER Hahm, SH Kim, KB Singh, L Nyiranshut, JD Latoche, CJ Anderson, J Adamik, DL Galson, KR Weiss, RJ Watters, B Lee, PN Kumta, SV Singh
Mol. Cancer Ther., 2019-11-29;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Egg White Ovotransferrin Attenuates RANKL-Induced Osteoclastogenesis and Bone Resorption
Authors: N Shang, J Wu
Nutrients, 2019-09-19;11(9):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis
Authors: L Li, AH Ameri, S Wang, KH Jansson, OM Casey, Q Yang, ML Beshiri, L Fang, RG Lake, S Agarwal, AN Alilin, W Xu, J Yin, K Kelly
Oncogene, 2019-07-16;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Global deletion of Optineurin results in altered type I IFN signaling and abnormal bone remodeling in a model of Paget's disease
Authors: SW Wong, BW Huang, X Hu, E Ho Kim, JP Kolb, RJ Padilla, P Xue, L Wang, TH Oguin, PA Miguez, HC Tseng, CC Ko, J Martinez
Cell Death Differ., 2019-05-10;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of cytoskeleton and adhesion signaling in osteoclasts by tetraspanin CD82
Authors: A Bergsma, SS Ganguly, ME Wiegand, D Dick, BO Williams, CK Miranti
Bone Rep, 2019-01-30;10(0):100196.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Sensory Neuropeptides and their Receptors Participate in Mechano-Regulation of Murine Macrophages
Authors: D Muschter, AS Beiderbeck, T Späth, C Kirschneck, A Schröder, S Grässel
Int J Mol Sci, 2019-01-24;20(3):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Trabectedin Reduces Skeletal Prostate Cancer Tumor Size in Association with Effects on M2 Macrophages and Efferocytosis
Authors: JD Jones, BP Sinder, D Paige, FN Soki, AJ Koh, S Thiele, Y Shiozawa, LC Hofbauer, S Daignault, H Roca, LK McCauley
Neoplasia, 2018-12-31;21(2):172-184.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Skeletal phenotype of the neuropeptide Y knockout mouse
Authors: NKY Wee, BP Sinder, S Novak, X Wang, C Stoddard, BG Matthews, I Kalajzic
Neuropeptides, 2018-11-30;73(0):78-88.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Inhibition of STAT5A promotes osteogenesis by DLX5 regulation
Authors: KM Lee, KH Park, JS Hwang, M Lee, DS Yoon, HA Ryu, HS Jung, KW Park, J Kim, SW Park, SH Kim, YM Chun, WJ Choi, JW Lee
Cell Death Dis, 2018-11-14;9(11):1136.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Bevacizumab or fibronectin gene editing inhibits the osteoclastogenic effects of fibroblasts derived from human radicular cysts
Authors: HC Wang, P Wang, YW Chen, Y Zhang
Acta Pharmacol. Sin., 2018-10-31;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
C5aR1 interacts with TLR2 in osteoblasts and stimulates the osteoclast-inducing chemokine CXCL10
Authors: Y Mödinger, A Rapp, J Pazmandi, A Vikman, K Holzmann, M Haffner-Lu, M Huber-Lang, A Ignatius
J. Cell. Mol. Med., 2018-09-24;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Artemisinin-Daumone Hybrid Inhibits Cancer Cell-Mediated Osteolysis by Targeting Cancer Cells and Osteoclasts
Authors: GT Ma, SK Lee, KK Park, J Park, SH Son, M Jung, WY Chung
Cell. Physiol. Biochem., 2018-09-11;49(4):1460-1475.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Modulating bone marrow hematopoietic lineage potential to prevent bone metastasis in breast cancer
Authors: JM Ubellacker, N Baryawno, N Severe, MJ DeCristo, J Sceneay, JN Hutchinson, MT Haider, CS Rhee, Y Qin, WM Gregory, AC Garrido-Ca, I Holen, JE Brown, RE Coleman, DT Scadden, SS McAllister
Cancer Res., 2018-07-31;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Substance P modulates bone remodeling properties of murine osteoblasts and osteoclasts
Authors: T Niedermair, S Schirner, R Seebröker, RH Straub, S Grässel
Sci Rep, 2018-06-15;8(1):9199.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Identification of a transporter complex responsible for the cytosolic entry of nitrogen-containing-bisphosphonates
Authors: Z Yu, LE Surface, CY Park, MA Horlbeck, GA Wyant, M Abu-Remail, TR Peterson, DM Sabatini, JS Weissman, EK O'Shea
Elife, 2018-05-10;7(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
OC-STAMP promotes osteoclast fusion for pathogenic bone resorption in periodontitis via up-regulation of permissive fusogen CD9
Authors: T Ishii, M Ruiz-Torru, A Ikeda, S Shindo, A Movila, H Mawardi, A Albassam, RA Kayal, AA Al-Dharrab, K Egashira, W Wisitrasam, K Yamamoto, AI Mira, K Sueishi, X Han, MA Taubman, T Miyamoto, T Kawai
FASEB J., 2018-03-13;0(0):fj201701424R.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The Long Pentraxin 3 Plays a Role in Bone Turnover and Repair
Authors: D Gr?evi?, M Sironi, S Valentino, L Deban, H Cvija, A Inforzato, N Kova?i?, V Katavi?, T Kelava, I Kalajzi?, A Mantovani, B Bottazzi
Front Immunol, 2018-03-05;9(0):417.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Monomeric C-Reactive Protein Binds and Neutralizes Receptor Activator of NF-?B Ligand-Induced Osteoclast Differentiation
Authors: ZK Jia, HY Li, YL Liang, LA Potempa, SR Ji, Y Wu
Front Immunol, 2018-02-19;9(0):234.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Adenosine Areceptor (A2AR) stimulation modulates expression of semaphorins 4D and 3A, regulators of bone homeostasis
Authors: A Mediero, T Wilder, L Shah, BN Cronstein
FASEB J., 2018-02-02;0(0):fj201700217R.
Species: Mouse
Sample Types: Whole Cells
Applications: Differentiation, Differentiation -
Targeted disruption of adenosine kinase in myeloid monocyte cells increases osteoclastogenesis and bone resorption in mice
Authors: Q Ye, G Li, S Liu, Y Guan, Y Li, J Li, H Jia, X Li, Q Li, R Huang, H Wang, Y Zhang
Int. J. Mol. Med., 2018-01-17;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Microbiota Reconstitution Does Not Cause Bone Loss in Germ-Free Mice
Authors: D Quach, F Collins, N Parameswar, L McCabe, RA Britton
mSphere, 2018-01-03;3(1):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The Effects of Kaempferol-Inhibited Autophagy on Osteoclast Formation
Authors: CJ Kim, SH Shin, BJ Kim, CH Kim, JH Kim, HM Kang, BS Park, IR Kim
Int J Mol Sci, 2018-01-02;19(1):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Effects of Osteogenic-Conditioned Medium from Human Periosteum-Derived Cells on Osteoclast Differentiation
Authors: HC Park, YB Son, SL Lee, GJ Rho, YH Kang, BW Park, SH Byun, SC Hwang, IA Cho, YC Cho, IY Sung, DK Woo, JH Byun
Int J Med Sci, 2017-11-02;14(13):1389-1401.
Species: Human
Sample Types: Cell Culture Supernates
-
Small leucine rich proteoglycans, a novel link to osteoclastogenesis
Authors: V Kram, TM Kilts, N Bhattachar, L Li, MF Young
Sci Rep, 2017-10-03;7(1):12627.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
SNX10 gene mutation leading to osteopetrosis with dysfunctional osteoclasts
Authors: EL Stattin, P Henning, J Klar, E McDermott, C Stecksen-B, PE Sandström, TG Kellgren, P Rydén, G Hallmans, T Lönnerholm, A Ameur, MH Helfrich, FP Coxon, N Dahl, J Wikström, UH Lerner
Sci Rep, 2017-06-07;7(1):3012.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Adipokine Chemerin Bridges Metabolic Dyslipidemia and Alveolar Bone Loss in Mice
Authors: Erivan S Ramos-Juni
J. Bone Miner. Res, 2017-03-01;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Inhibition of lipopolysaccharide-induced osteoclast formation and bone resorption in vitro and in vivo by cysteine proteinase inhibitors
Authors: F Strålberg, A Kassem, F Kasprzykow, M Abrahamson, A Grubb, C Lindholm, UH Lerner
J. Leukoc. Biol, 2017-02-14;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Dynamics of the sealing zone in cultured osteoclasts
Authors: Sarit Batsir
Cytoskeleton (Hoboken), 2017-02-08;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Tumor Cell-Derived Exosomes from the Prostate Cancer Cell Line TRAMP-C1 Impair Osteoclast Formation and Differentiation
PLoS ONE, 2016-11-10;11(11):e0166284.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Prader-Willi Critical Region, a Non-Translated, Imprinted Central Regulator of Bone Mass: Possible Role in Skeletal Abnormalities in Prader-Willi Syndrome.
Authors: Khor E, Fanshawe B, Qi Y, Zolotukhin S, Kulkarni R, Enriquez R, Purtell L, Lee N, Wee N, Croucher P, Campbell L, Herzog H, Baldock P
PLoS ONE, 2016-01-29;11(1):e0148155.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Heavy Metal Ion Regulation of Gene Expression: MECHANISMS BY WHICH LEAD INHIBITS OSTEOBLASTIC BONE-FORMING ACTIVITY THROUGH MODULATION OF THE Wnt/beta-CATENIN SIGNALING PATHWAY.
Authors: Beier E, Sheu T, Dang D, Holz J, Ubayawardena R, Babij P, Puzas J
J Biol Chem, 2015-05-14;290(29):18216-26.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Netrin-1 is a critical autocrine/paracrine factor for osteoclast differentiation.
Authors: Mediero A, Ramkhelawon B, Perez-Aso M, Moore K, Cronstein B
J Bone Miner Res, 2015-05-01;30(5):837-54.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Activin A inhibits RANKL-mediated osteoclast formation, movement and function in murine bone marrow macrophage cultures.
Authors: Fowler T, Kamalakar A, Akel N, Kurten R, Suva L, Gaddy D
J Cell Sci, 2015-01-20;128(4):683-94.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of osteoclast homeostasis and inflammatory bone loss by MFG-E8.
Authors: Abe T, Shin J, Hosur K, Udey M, Chavakis T, Hajishengallis G
J Immunol, 2014-06-23;193(3):1383-91.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Notch pathway inhibition controls myeloma bone disease in the murine MOPC315.BM model.
Authors: Schwarzer R, Nickel N, Godau J, Willie B, Duda G, Schwarzer R, Cirovic B, Leutz A, Manz R, Bogen B, Dorken B, Jundt F
Blood Cancer J, 2014-06-13;4(0):e217.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
NOTCH inhibits osteoblast formation in inflammatory arthritis via noncanonical NF-kappaB.
Authors: Zhang H, Hilton M, Anolik J, Welle S, Zhao C, Yao Z, Li X, Wang Z, Boyce B, Xing L
J Clin Invest, 2014-06-02;124(7):3200-14.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A nonapoptotic role for CASP2/caspase 2: modulation of autophagy.
Authors: Tiwari, Meenaksh, Sharma, Lokendra, Vanegas, Difernan, Callaway, Danielle, Bai, Yidong, Lechleiter, James D, Herman, Brian
Autophagy, 2014-06-01;10(6):1054-70.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TBK1 mediates critical effects of measles virus nucleocapsid protein (MVNP) on pagetic osteoclast formation.
Authors: Sun Q, Sammut B, Wang F, Kurihara N, Windle J, Roodman G, Galson D
J Bone Miner Res, 2014-01-01;29(1):90-102.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation.
Authors: Xiu Y, Xu H, Zhao C, Li J, Morita Y, Yao Z, Xing L, Boyce B
J Clin Invest, 2013-12-09;124(1):297-310.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Osteoblast CFTR inactivation reduces differentiation and osteoprotegerin expression in a mouse model of cystic fibrosis-related bone disease.
Authors: Stalvey M, Clines K, Havasi V, McKibbin C, Dunn L, Chung W, Clines G
PLoS ONE, 2013-11-13;8(11):e80098.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Chemerin neutralization blocks hematopoietic stem cell osteoclastogenesis.
Authors: Muruganandan S, Dranse H, Rourke J, McMullen N, Sinal C
Stem Cells, 2013-10-01;31(10):2172-82.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Macrophage infiltration predicts a poor prognosis for human ewing sarcoma.
Authors: Fujiwara T, Fukushi J, Yamamoto S, Matsumoto Y, Setsu N, Oda Y, Yamada H, Okada S, Watari K, Ono M, Kuwano M, Kamura S, Iida K, Okada Y, Koga M, Iwamoto Y
Am. J. Pathol., 2011-07-21;179(3):1157-70.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE.
Authors: Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J
Calcif. Tissue Int., 2010-08-20;87(5):461-8.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis.
Authors: Binder NB, Niederreiter B, Hoffmann O, Stange R, Pap T, Stulnig TM, Mack M, Erben RG, Smolen JS, Redlich K
Nat. Med., 2009-03-29;15(4):417-24.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay, Flow Cytometry -
Hematopoietic stem cell-targeted neonatal gene therapy reverses lethally progressive osteopetrosis in oc/oc mice.
Authors: Johansson MK, de Vries TJ, Schoenmaker T, Ehinger M, Brun AC, Fasth A, Karlsson S, Everts V, Richter J
Blood, 2007-03-01;109(12):5178-85.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Gingival fibroblasts are better at inhibiting osteoclast formation than periodontal ligament fibroblasts.
Authors: de Vries TJ, Schoenmaker T, Wattanaroonwong N, van den Hoonaard M, Nieuwenhuijse A, Beertsen W, Everts V
J. Cell. Biochem., 2006-05-15;98(2):370-82.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone.
Authors: Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA, Parisien MV, Kim W, Winchester RJ, Lee FY
J. Orthop. Res., 2005-01-01;23(1):203-9.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Mouse TRANCE/RANK L/TNFSF11 (E. coli-expressed)
Average Rating: 4.8 (Based on 14 Reviews)
Have you used Recombinant Mouse TRANCE/RANK L/TNFSF11 (E. coli-expressed)?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Reason for Rating: Works well on in vitro osteoclast differentiation exp.
Reason for Rating: Steady quality! 50 ng/ml RANKL can induce RAW264.7 cells to osteoclast very well.
Reason for Rating: Using R&D RANKL gave me what I was expecting. Large multinucleated osteoclasts appeared in the culture on day 5. RANKL with added stabilizer (BSA) is another advantage. Highly recommended.
Reason for Rating: The RANKL from RnD works excellent.
Reason for Rating: I used 10ng/mL to treat the isolated mouse macrophage cells. Works good.
Reason for Rating: The R&D RANKL is the best for osteoclast differentiation.
Reason for Rating: I use 10ng to treat the primary murine macrophage. It works good.
Reason for Rating: 15ng/ml works very well
Reason for Rating: This is the only Rank L we use. It has been prove to work effectively time and time again. We use it to active our cells into Osteoclasts.
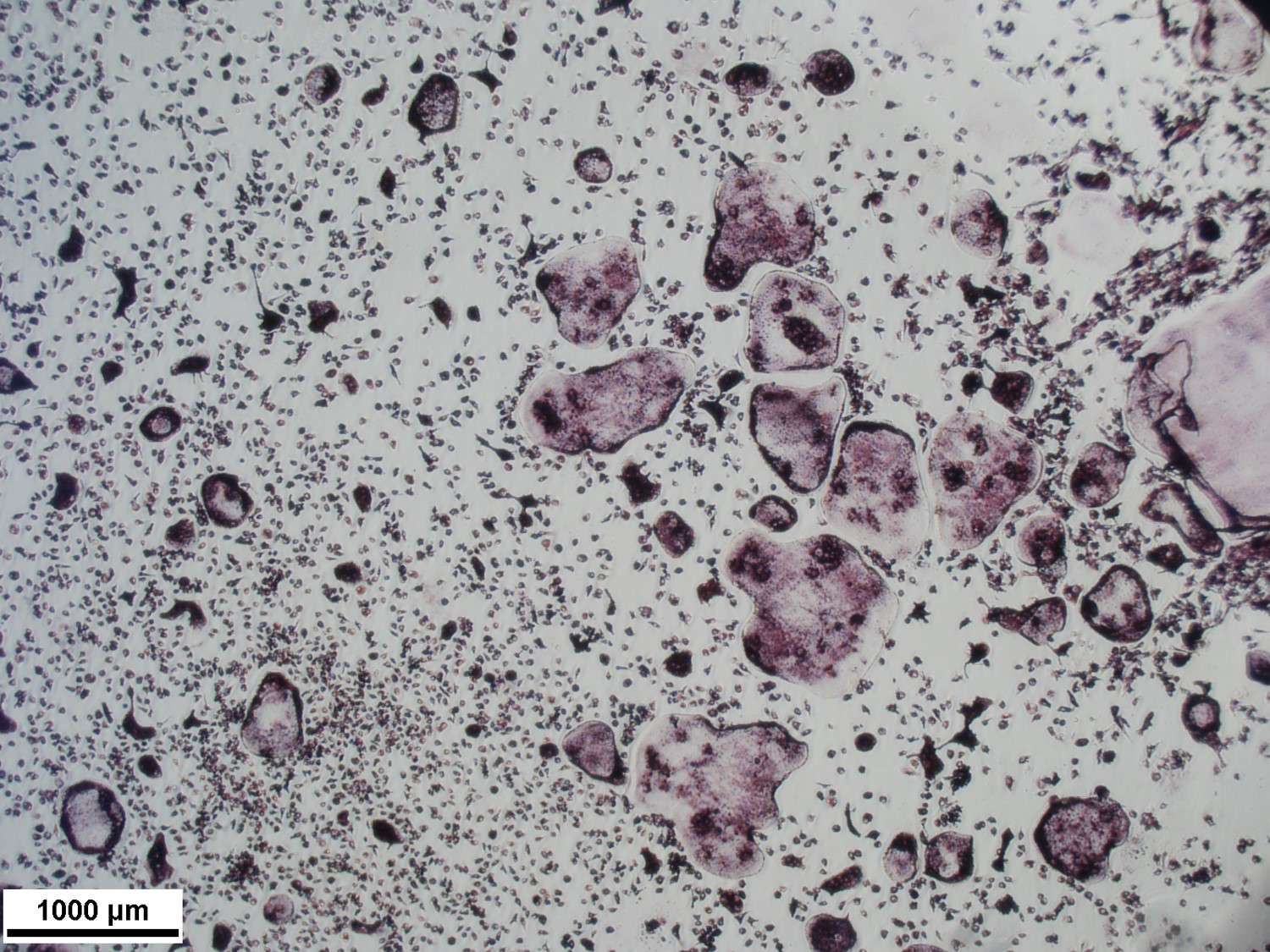
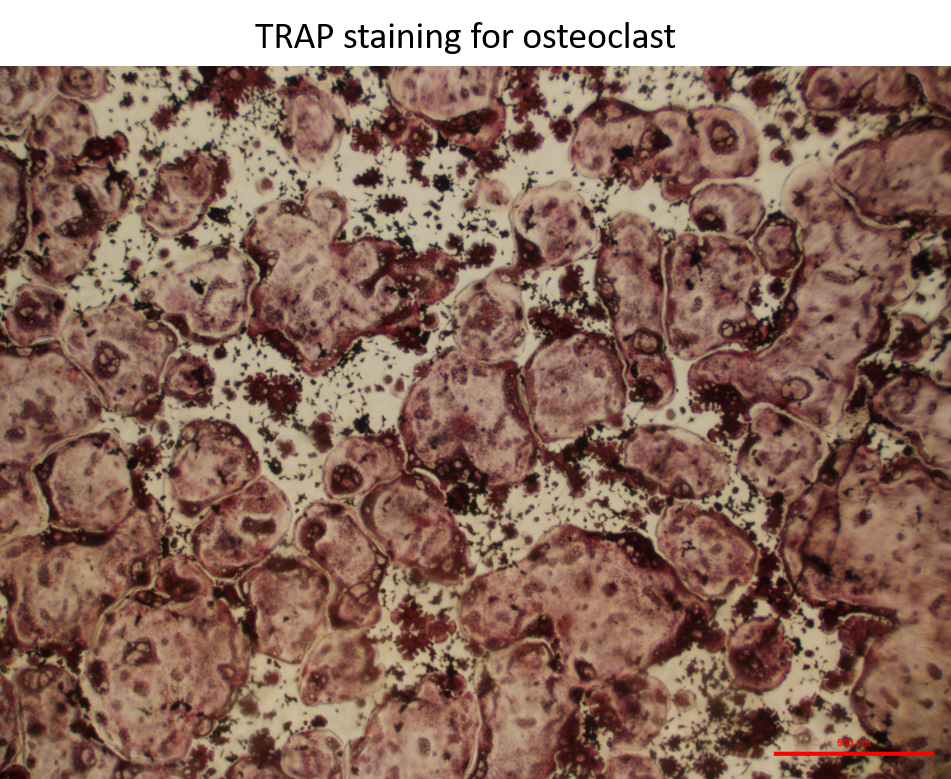
Bone marrow macrophages grown in media on osteoassay plates for 7 days then treated with 35ng/mL Rank L. After 14 days TRAP stained.