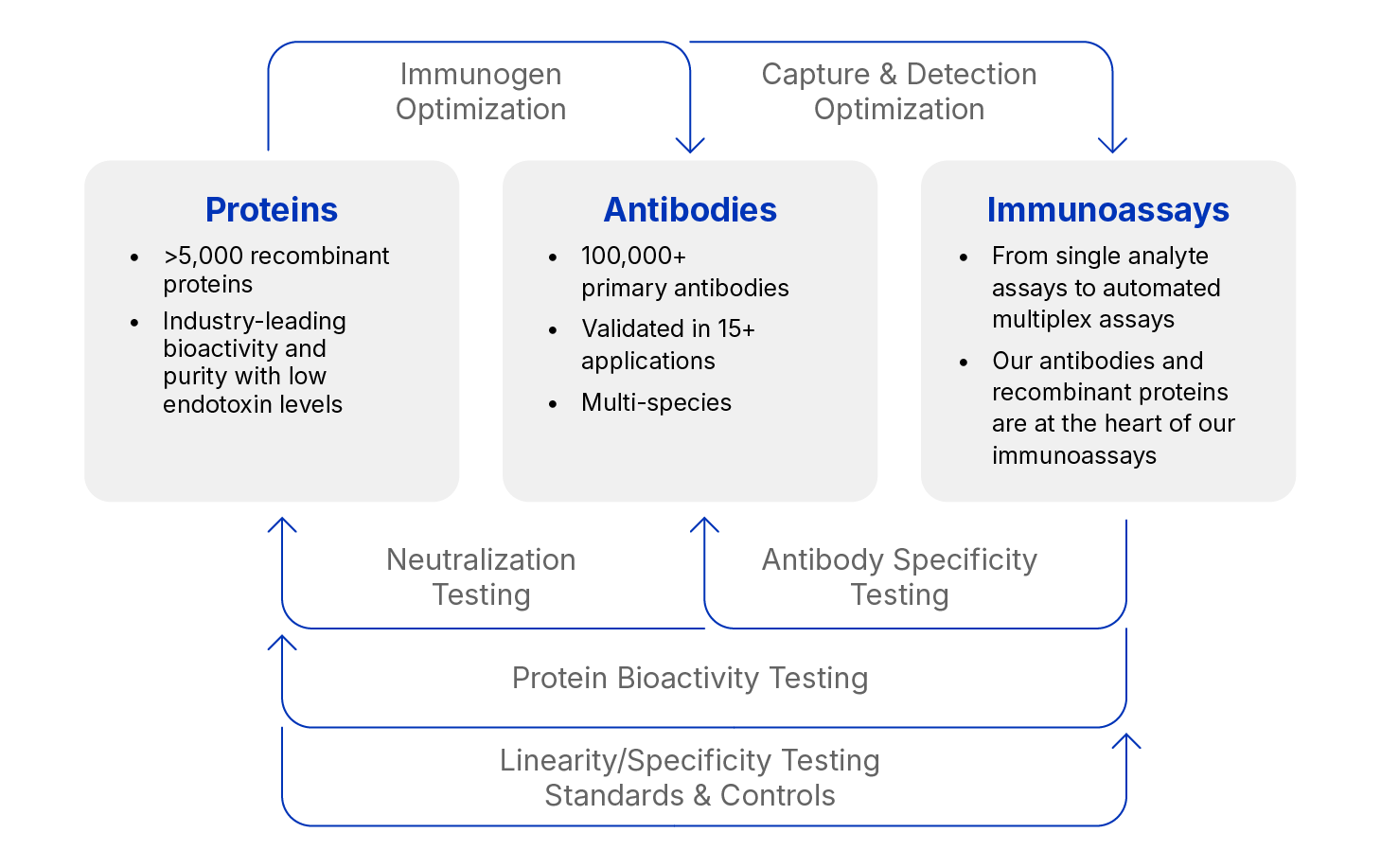
Recombinant Protein Quality – Protein Production
Quality is our number one priority. We manufacture more than 95% of our proteins at our facility. This means we have complete control over all quality testing and nothing becomes a product that does not meet our industry-leading specifications. A highly trained technical service staff and the scientists responsible for protein development are on site to answer any questions you might have. R&D Systems’ experience and dedication to protein development and manufacturing is unmatched in the life science industry.
The Road to Research Success
This infographic shows how Bio-Techne products, and our consistency to quality, will help you advance your research efforts. What tools are you using in your research? Good results are everything. Trust your reagents
Lot-to-Lot Consistency: Ensuring experiments can always be repeated!
- For new proteins multiple lots are created, all with matching specifications. This ensures that there will be no problem matching original lots in the future.
- Each new lot is tested side-by-side with previous lots for purity, biological potency, and endotoxin level.
Biological Activity
- Proprietary methods for accurate protein folding ensure biologically relevant proteins
- R&D systems uses over 900 validated bioassays to evaluate recombinant proteins bioactivity
- Bioactivity is measured experimentally with the appropriate biological system.
Endotoxin Level and Microbial Bioburden Test
- Each new production lot of protein is assessed for endotoxin using the Limulus Amoebocyte Lysates (LAL) assay. The endotoxin specification is an industry-leading <0.1 EU/ug.
- Each protein is tested to rule out microbial contamination using direct plating and broth dilution, according to guidelines from the United States Pharmacopeia (USP).
Protein Sequence Accuracy
- N-terminal amino acid sequence analysis is performed for each recombinant protein to verify the protein produced matches the sequence expected
Formulation
- Recombinant proteins are formulated for best stability and are lyophilized from a sterile filtered solution. They are available without or with a carrier protein, usually bovine serum albumin (BSA) at 50 mg/mg of protein.
- Recombinant enzymes are in solution and ready to use
