Resveratrol
Tocris Bioscience | Catalog # 1418

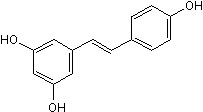
Product Description
Resveratrol is a phytoestrogen with antitumor, antioxidant, antiplatelet, anti-inflammatory and antifungal effects. Inhibits cytochrome P450 1A1 (IC50 = 23 μM) and displays mixed agonist/antagonist actions at ERα and ERβ estrogen receptors. Converted into the anticancer agent piceatannol (Cat. No. 1554) by cytochrome P450 1B1. Activates autophagy. Also activates TRPA1 in prostate cancer-associated fibroblasts. Also SIRT1 activator.
Product Specifications for Resveratrol
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 22.82 | 100 | |
| Ethanol | 100 |
Preparing Stock Solutions for Resveratrol
The following data is based on the product molecular weight 228.25.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 4.38 mL | 21.91 mL | 43.81 mL |
| 5 mM | 0.88 mL | 4.38 mL | 8.76 mL |
| 10 mM | 0.44 mL | 2.19 mL | 4.38 mL |
| 50 mM | 0.09 mL | 0.44 mL | 0.88 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 45 publications citing the usage of this product.
- Vancauwenberghe Activation of mutated TRPA1 ion channel by resveratrol in human prostate cancer associated fibroblasts (CAF). Mol.Carcinog. 2017 PMID: 28277613
- Baptista Regulation of histone H2A.Z exprsesion is mediated by sirtuin 1 in prostate cancer. Oncotarget 2013 PMID: 24127549
- Galluzzi Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat.Rev.Drug.Discov. 2017 PMID: 28529316
- Kimura Effects of stilbenes on arachidonate metabolism in leukocytes. Biochim.Biophys.Acta 1985 PMID: 3922423
- Fremont Biological effects of resveratrol. Life Sci. 2000 PMID: 10680575
- Chun Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem.Biophys.Res.Commun. 1999 PMID: 10448061
- Bowers Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and β. Endocrinology 2000 PMID: 11014220
- Baur and Sinclair Therapeutic potential of resveratrol: the in vivo evidence. Nat.Rev.Drug Discov. 2006 PMID: 16732220
Product Documents for Resveratrol
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Resveratrol
For research use only
Related Research Areas
Citations for Resveratrol
Customer Reviews for Resveratrol (1)
Have you used Resveratrol?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Species: HumanAssay Type: In VitroCell Line/Tissue: human retinal pigment epithelial (ARPE)Verified Customer | Posted 09/06/2025I tested the protective effects of resveratrol against cell death induced by hyper-glycemia in human retinal pigment epithelial (ARPE).
There are no reviews that match your criteria.

