Ro 61-8048
Tocris Bioscience | Catalog # 3254

Key Product Details
Description
Product Description
Ro 61-8048 is a potent and competitive kynurenine 3-monooxygenase (kynurenine 3-hydroxylase; KMO) inhibitor (Ki = 4.8 nM, IC50 = 37 nM). Increases kynurenic acid levels to concentrations that antagonize the glycine site of NMDA receptors. Brain penetrant and exhibits antidystonic, anticonvulsant and neuroprotective activities. Ro 61-8048 decreases nicotine self-administration in vivo. Ro 61-8048 prevents post-operative brain edema and consequent neuronal apoptosis in a rat model of surgically induced brain injury.
Product Specifications for Ro 61-8048
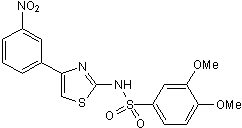
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 42.15 | 100 | |
| Ethanol | 4.21 | 10 |
Preparing Stock Solutions for Ro 61-8048
The following data is based on the product molecular weight 421.45.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 2.37 mL | 11.86 mL | 23.73 mL |
| 5 mM | 0.47 mL | 2.37 mL | 4.75 mL |
| 10 mM | 0.24 mL | 1.19 mL | 2.37 mL |
| 50 mM | 0.05 mL | 0.24 mL | 0.47 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 9 publications citing the usage of this product.
- Zakhary Modification of kynurenine pathway via inhibition of kynurenine hydroxylase attenuates surgical brain injury complications in a male rat model. J Neurosci Res 2020 PMID: 31257634
- Secci Attenuating nicotine reinforcement and relapse by enhancing endogenous brain levels of kynurenic acid in rats and squirrel monkeys. Neuropsychopharmacology 2017 PMID: 28139681
- Justinova Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat.Neurosci. 2013 PMID: 24121737
- Hamann Effects of kynurenine 3-hydroxylase inhibitor Ro 61-8048 after intrastriatal injections on the severity of dystonia in the dtsz mutant. Eur.J.Pharmacol. 2008 PMID: 18353306
- Carpenedo Kynurenine 3-mono-oxygenase inhibitors attenuate post-ischaemic neuronal death in organotypic hippocampal slice cultures. J.Neurochem. 2002 PMID: 12354294
- Rover Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high affinity inhibitors of kynurenine 3-hydroxylase. J.Med.Chem. 1997 PMID: 9435907
Product Documents for Ro 61-8048
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Ro 61-8048
For research use only
Related Research Areas
Citations for Ro 61-8048
Customer Reviews for Ro 61-8048
There are currently no reviews for this product. Be the first to review Ro 61-8048 and earn rewards!
Have you used Ro 61-8048?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
