Rosiglitazone
Tocris Bioscience | Catalog # 5325

Key Product Details
Description
Alternative Names
Product Description
Rosiglitazone is a potent and selective PPARγ agonist (EC50 = 60 nM); exhibits no activity at PPARα and PPARβ. Promotes differentiation of pluripotent C3H10T1/2 stem cells into adipocytes. Also promotes differentiation of urothelial organoids in combination with Erlotinib (Cat. No. 7194). Exhibits antihyperglycemic activity in diabetic ob/ob mouse model. Antidiabetic agent.
Product Specifications for Rosiglitazone
Molecular Weight
Formula
Storage
Purity
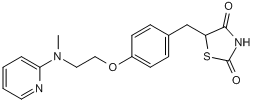
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 35.74 | 100 |
Preparing Stock Solutions for Rosiglitazone
The following data is based on the product molecular weight 357.43.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 2.80 mL | 13.99 mL | 27.98 mL |
| 5 mM | 0.56 mL | 2.80 mL | 5.60 mL |
| 10 mM | 0.28 mL | 1.40 mL | 2.80 mL |
| 50 mM | 0.06 mL | 0.28 mL | 0.56 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 55 publications citing the usage of this product.
- Willson The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J.Med.Chem. 1996 PMID: 8576907
- Santos Urothelial organoids originating from Cd49fhigh mouse stem cells display Notch-dependent differentiation capacity. Nat.Commun. 2019 PMID: 31562298
- Lehmann An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J.Biol.Chem. 1995 PMID: 7768881
Product Documents for Rosiglitazone
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Rosiglitazone
For research use only
Related Research Areas
Citations for Rosiglitazone
Customer Reviews for Rosiglitazone (1)
Have you used Rosiglitazone?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
-
Species: HumanAssay Type: In VitroCell Line/Tissue: PPARg reporter cellsVerified Customer | Posted 12/07/2019PPAR gamma Assay was performed using manual dispensing and following the protocol described in this Technical Manual, using the Rosiglitazone. PPAR gamma reporter cells treated with 2,500 nM Rosiglitazone yielded an average RLU value with CV=7%, S/B = 162 and a corresponding Z’= 0.78.
There are no reviews that match your criteria.
