Taxol
Tocris Bioscience | Catalog # 1097

Key Product Details
Description
Alternative Names
Product Description
Taxol, also known as paclitaxel, promotes and stabilizes tubulin polymerization, causing cell cycle arrest. In CCRF-HSB-2 cells, Taxol induces autocatalytic activation of caspase-10, triggering apoptosis. Taxol is an antitumor agent. In a mouse model of bladder cancer Taxol decreases tumor growth. Taxol also prolongs survival of mice transplanted with human ovarian carcinoma xenografts. Taxol improves neurological outcome and magnetic resonance imaging biomarkers after traumatic brain injury in mice.
Product Specifications for Taxol
Molecular Weight
Formula
Storage
Purity
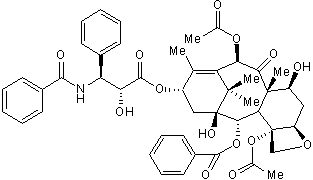
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 85.39 | 100 with gentle warming | |
| Ethanol | 21.35 | 25 with gentle warming |
Preparing Stock Solutions for Taxol
The following data is based on the product molecular weight 853.92.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 1.17 mL | 5.86 mL | 11.71 mL |
| 5 mM | 0.23 mL | 1.17 mL | 2.34 mL |
| 10 mM | 0.12 mL | 0.59 mL | 1.17 mL |
| 50 mM | 0.02 mL | 0.12 mL | 0.23 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 128 publications citing the usage of this product.
- Nicoletti Antitumor activity of Tax. (NSC-125973) in human ovarian carcinomas growing in the peritoneal cavity of nude mice. Ann.Oncol. 1993 PMID: 8095399
- Cross PacT improves outcome from traumatic brain injury. Brain Res. 2015 PMID: 26086366
- Rao Characterization of the Tax. binding site on the microtubule. J.Biol.Chem. 1999 PMID: 10608867
- Park Taxol induces caspase-10-dependent apoptosis. J.Biol.Chem. 2004 PMID: 15452117
- McGuire Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann.Intern.Med. 1989 PMID: 2569287
- Rowinsky Taxol: a novel investigational antimicrotubule agent. J.Natl.Cancer Inst. 1990 PMID: 1973737
Product Documents for Taxol
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Taxol
For research use only
⚠ WARNING: This product can expose you to chemicals including Paclitaxel, which is known to the State of California to cause reproductive toxicity with developmental effects. For more information, go to www.P65Warnings.ca.govRelated Research Areas
Citations for Taxol
Customer Reviews for Taxol (2)
Have you used Taxol?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Species: HumanAssay Type: In VitroVerified Customer | Posted 10/13/2022Taxol was used for the establishment of taxol-resistant pancreatic cancer cell lines.
-
Species: MouseAssay Type: In VivoCell Line/Tissue: Human epidermal carcinoma cell lineVerified Customer | Posted 11/17/2019Human epidermal carcinoma cell line was treated with Paclitaxel, docetaxel, vincristine, vinblastine, colchicine, cisplatin, and mitoxantrone which purchased from Tocris Bioscience.
There are no reviews that match your criteria.

