Brefeldin A
Tocris Bioscience | Catalog # 1231

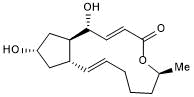
Key Product Details
Description
Product Description
Brefeldin A is a reversible inhibitor of protein translocation from the endoplasmic reticulum (ER) to the Golgi apparatus. Blocks binding of ADP-ribosylation factor (ARF1) to the Golgi apparatus and inhibits GDP-GTP exchange, leading to activation of ER stress signaling pathways. Can be used to induce autophagy in mammalian cells. Also enhances CRISPR-mediated homology-directed repair (HDR) efficiency ~2-fold when applied at 100 nM, in human induced pluripotent stem cells (iPSCs). Antifungal.
Product Specifications for Brefeldin A
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 14.02 | 50 |
Preparing Stock Solutions for Brefeldin A
The following data is based on the product molecular weight 280.36.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 0.5 mM | 7.13 mL | 35.67 mL | 71.34 mL |
| 2.5 mM | 1.43 mL | 7.13 mL | 14.27 mL |
| 5 mM | 0.71 mL | 3.57 mL | 7.13 mL |
| 25 mM | 0.14 mL | 0.71 mL | 1.43 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 95 publications citing the usage of this product.
- Oh-Hashi Elucidation of brefeldin A-induced ER and Golgi stress responses in Neuro2a cells. Mol. Cell. Biochem. 2021 PMID: 34129155
- Yu Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell Stem Cell 2015 PMID: 25658371
- Ding Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J.Biol.Chem. 2007 PMID: 17135238
- Tsai Identification of a brefeldin A-insensitive guanine nucleotide-exchange protein for ADP-ribosylation factor in bovine brain. Proc.Natl.Acad.Sci.U.S.A. 1994 PMID: 8159707
- Morinaga Isolation of a brefeldin A-inhibited guanine nucleotide-exchange protein for ADP ribosylation factor (ARF)1 and ARF3 that contains a Sec7-like domain. Proc.Natl.Acad.Sci.U.S.A. 1996 PMID: 8917509
- Ktistakis Action of brefeldin A blocked by activation of a pertussis-toxin-sensitive G protein. Nature 1992 PMID: 1549178
Product Documents for Brefeldin A
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Brefeldin A
For research use only
Citations for Brefeldin A
Customer Reviews for Brefeldin A (1)
Have you used Brefeldin A?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Species: HumanAssay Type: In VitroCell Line/Tissue: KMS11 or OPM2Verified Customer | Posted 06/20/2020We treated cells for 16 hr.0.2 μg/ml
There are no reviews that match your criteria.

