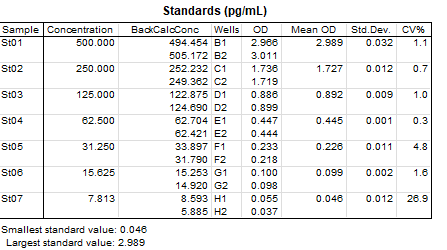
Human GM-CSF Quantikine ELISA Kit Summary
Sample Values
Serum/Plasma - Forty serum and plasma samples from apparently healthy volunteers were evaluated for the presence of human GM-CSF in this assay. All samples measured less than the lowest Human GM-CSF Standard, 7.8 pg/mL. No medical histories were available for the donors used in this study.| Condition | Day 1 (pg/mL) | Day 5 (pg/mL) |
| Unstimulated | 39 | 43 |
| Stimulated | 71 | 31 |
Product Summary
Precision
Cell Culture Supernates
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 46.8 | 131 | 269 | 45.1 | 133 | 266 |
| Standard Deviation | 1.1 | 3.6 | 7.3 | 2.4 | 7 | 11.5 |
| CV% | 2.4 | 2.7 | 2.7 | 5.3 | 5.3 | 4.3 |
Serum, EDTA Plasma, Heparin Plasma, Citrate Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 54.7 | 156 | 321 | 53.1 | 157 | 317 |
| Standard Deviation | 1.1 | 3.2 | 4.8 | 3.3 | 9.1 | 15.4 |
| CV% | 2 | 2.1 | 1.5 | 6.2 | 5.8 | 4.9 |
Recovery
The recovery of GM-CSF spiked to three different levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=5) | 97 | 85-104 |
| Citrate Plasma (n=5) | 101 | 92-106 |
| EDTA Plasma (n=5) | 100 | 94-105 |
| Heparin Plasma (n=5) | 98 | 92-104 |
| Serum (n=5) | 98 | 93-103 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: GM-CSF
GM-CSF (Granulocyte-Macrophage Colony Stimulating Factor) induces the development of monocytes, neutrophils, eosinophils, and myeloid and dermal dendritic cells. It also acts as a neutrophil and dendritic cell chemoattractant. GM-CSF promotes Th1 and Th17 cell mediated autoimmune inflammation as well as the inflammatory activation of dendritic cells, microglia, alveolar macrophages, and eosinophils. In addition, it cooperates with G-CSF in promoting tumor cell proliferation and invasion. GM-CSF signals through a receptor complex composed of GM-CSF R alpha and the Common beta Chain (beta c) with Syndecan-2 as a potential co-receptor. The beta c subunit is shared by the receptor complexes for IL-3 and IL-5.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 100 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 200 µL of Conjugate to each well.
- For Serum & Plasma Samples: Cover with a new plate sealer, and incubate at room temperature for 2 hours.
For Cell Culture Supernate Samples: Cover with a new plate sealer, and incubate at room temperature for 1 hour. - Aspirate and wash 4 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 20 minutes. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human GM-CSF Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
59
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Generation of induced alveolar assembloids with functional alveolar-like macrophages
Authors: Kang, JS;Lee, Y;Lee, Y;Gil, D;Kim, MJ;Wood, C;Delorme, V;Lee, JM;Ko, KC;Kim, JH;Lee, MO;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Migration arrest and transendothelial trafficking of human pathogenic-like Th17 cells are mediated by differentially positioned chemokines
Authors: Parween, F;Singh, SP;Kathuria, N;Zhang, HH;Ashida, S;Otaizo-Carrasquero, FA;Shamsaddini, A;Gardina, PJ;Ganesan, S;Kabat, J;Lorenzi, HA;Riley, DJ;Myers, TG;Pittaluga, S;Bielekova, B;Farber, JM;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Chemokine positioning determines mutually exclusive roles for their receptors in extravasation of pathogenic human T cells
Authors: F Parween, SP Singh, HH Zhang, N Kathuria, FA Otaizo-Car, A Shamsaddin, PJ Gardina, S Ganesan, J Kabat, HA Lorenzi, TG Myers, JM Farber
bioRxiv : the preprint server for biology, 2023-02-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum granulocyte-macrophage colony-stimulating factor (GM-CSF) is increased in patients with active radiographic axial spondyloarthritis and persists despite anti-TNF treatment
Authors: C Papagoras, S Tsiami, A Chrysantho, I Mitroulis, X Baraliakos
Arthritis Research & Therapy, 2022-08-16;24(1):195.
Species: Human
Sample Types: Serum
-
Mesothelin?specific T cell cytotoxicity against triple negative breast cancer is enhanced by 40s ribosomal protein subunit 3?treated self?differentiated dendritic cells
Authors: N Jirapongwa, S Thongchot, W Chiraphapp, T Chieochans, D Sa-Nguanra, M Warnnissor, P Thuwajit, PT Yenchitsom, C Thuwajit
Oncology reports, 2022-05-26;48(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Evaluation of the alpha-casein (CSN1S1) locus as a potential target for a site-specific transgene integration
Authors: AV Smirnov, GV Kontsevaya, TA Shnaider, AM Yunusova, NA Feofanova, LA Gerlinskay, IA Serova, OL Serov, NR Battulin
Scientific Reports, 2022-05-14;12(1):7983.
Species: Mouse
Sample Types: Milk
-
miR-124-3p relieves allergic rhinitis by inhibiting dipeptidyl peptidase-4
Authors: S Zhang, D Dong, Y Zhang, J Wang, L Liu, Y Zhao
International immunopharmacology, 2021-10-26;101(0):108279.
Species: Human
Sample Types: Cell Culture Supernates
-
Development of mesothelin-specific CAR NK-92 cells for the treatment of gastric cancer
Authors: B Cao, M Liu, J Huang, J Zhou, J Li, H Lian, W Huang, Y Guo, S Yang, L Lin, M Cai, C Zhi, J Wu, L Liang, Y Hu, H Hu, J He, B Liang, Q Zhao, K Zhu
International journal of biological sciences, 2021-09-03;17(14):3850-3861.
Species: Human
Sample Types: Cell Culture Supernates
-
Psoralen inhibits the inflammatory response and mucus production in allergic rhinitis by inhibiting the activator protein�1 pathway and the downstream expression of cystatin?SN
Authors: W Gao, Z Jin, Y Zheng, Y Xu
Molecular Medicine Reports, 2021-07-19;24(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
miR-31 attenuates murine allergic rhinitis by suppressing interleukin-13-induced nasal epithelial inflammatory responses
Authors: F Zhou, P Liu, H Lv, Z Gao, W Chang, Y Xu
Mol Med Rep, 2020-11-12;23(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Inhibition of purinergic P2X receptor 7 (P2X7R) decreases granulocyte-macrophage colony-stimulating factor (GM-CSF) expression in U251 glioblastoma cells
Authors: M Drill, KL Powell, LK Kan, NC Jones, TJ O'Brien, JA Hamilton, M Monif
Sci Rep, 2020-09-09;10(1):14844.
Species: Human
Sample Types: Cell Culture Supernates
-
Suppressive effects of S100A8 and S100A9 on neutrophil apoptosis by cytokine release of human bronchial epithelial cells in asthma
Authors: DH Kim, A Gu, JS Lee, EJ Yang, A Kashif, MH Hong, G Kim, BS Park, SJ Lee, IS Kim
Int J Med Sci, 2020-02-04;17(4):498-509.
Species: Human
Sample Types: Cell Culture Supernates
-
Multilevel defects in the hematopoietic niche in essential thrombocythemia
Authors: T Sun, M Ju, X Dai, H Dong, W Gu, Y Gao, R Fu, X Liu, Y Huang, W Liu, Y Chi, W Wang, H Li, Y Zhou, L Shi, R Yang, L Zhang
Haematologica, 2019-07-09;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Sanshool improves UVB-induced skin photodamage by targeting JAK2/STAT3-dependent autophagy
Authors: D Hao, X Wen, L Liu, L Wang, X Zhou, Y Li, X Zeng, G He, X Jiang
Cell Death Dis, 2019-01-08;10(1):19.
Species: Human
Sample Types: Cell Culture Supernates
-
Glucocorticoid insensitivity by staphylococcal enterotoxin B in keratinocytes of allergic dermatitis is associated with impaired nuclear translocation of the Glucocorticoid Receptor ?
Authors: K Huang, L Ran, W Wang, R Zhou, X Cai, R Li, Y Li, C Zhou, W He, R Wang
J. Dermatol. Sci., 2018-11-26;92(3):272-280.
Species: Human
Sample Types: Cell Culture Supernates
-
Arming oncolytic reovirus with GM-CSF gene to enhance immunity
Authors: V Kemp, DJM van den Wo, MGM Camps, T van Hall, P Kinderman, N Pronk-van, RC Hoeben
Cancer Gene Ther., 2018-11-23;0(0):.
-
Occupational exposure to ultrafine particles in police officers: no evidence for adverse respiratory effects
Authors: G Jordakieva, I Grabovac, E Valic, KE Schmidt, A Graff, A Schuster, K Hoffmann-S, C Oberhuber, O Scheiner, A Goll, J Godnic-Cva
J Occup Med Toxicol, 2018-02-03;13(0):5.
Species: Human
Sample Types: Serum
Applications: ELISA Capture -
Adipose progenitor cell secretion of GM-CSF and MMP9 promotes a stromal and immunological microenvironment that supports breast cancer progression
Authors: F Reggiani, V Labanca, P Mancuso, C Rabascio, G Talarico, S Orecchioni, A Manconi, F Bertolini
Cancer Res., 2017-07-28;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Overexpression of GLP-1 receptors suppresses proliferation and cytokine release by airway smooth muscle cells of patients with chronic obstructive pulmonary disease via activation of ABCA1
Authors: YH Sun, L He, MY Yan, RQ Zhao, B Li, F Wang, Y Yang, HP Yu
Mol Med Rep, 2017-05-25;0(0):.
-
Association between Placental Lesions, Cytokines and Angiogenic Factors in Pregnant Women with Preeclampsia
Authors: Ingrid C Weel
PLoS ONE, 2016-06-17;11(6):e0157584.
Species: Human
Sample Types: Tissue Homogenates
-
The Novel Receptor C5aR2 Is Required for C5a-Mediated Human Mast Cell Adhesion, Migration, and Proinflammatory Mediator Production.
Authors: Pundir P, MacDonald C, Kulka M
J Immunol, 2015-08-17;195(6):2774-87.
Species: Human
Sample Types: Cell Culture Supernates
-
Sphingosine 1 Phosphate at the Blood Brain Barrier: Can the Modulation of S1P Receptor 1 Influence the Response of Endothelial Cells and Astrocytes to Inflammatory Stimuli?
Authors: Spampinato S, Obermeier B, Cotleur A, Love A, Takeshita Y, Sano Y, Kanda T, Ransohoff R
PLoS ONE, 2015-07-21;10(7):e0133392.
Species: Human
Sample Types: Cell Culture Supernates
-
Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer.
Authors: Park S, Breitbach C, Lee J, Park J, Lim H, Kang W, Moon A, Mun J, Sommermann E, Maruri Avidal L, Patt R, Pelusio A, Burke J, Hwang T, Kirn D, Park Y
Mol Ther, 2015-06-15;23(9):1532-40.
Species: Human
Sample Types: Plasma
-
High EMT Signature Score of Invasive Non-Small Cell Lung Cancer (NSCLC) Cells Correlates with NFkappaB Driven Colony-Stimulating Factor 2 (CSF2/GM-CSF) Secretion by Neighboring Stromal Fibroblasts.
Authors: Rudisch A, Dewhurst M, Horga L, Kramer N, Harrer N, Dong M, van der Kuip H, Wernitznig A, Bernthaler A, Dolznig H, Sommergruber W
PLoS ONE, 2015-04-28;10(4):e0124283.
Species: Human
Sample Types: Cell Culture Supernates
-
Autophagy promotes radiation-induced senescence but inhibits bystander effects in human breast cancer cells.
Authors: Huang, Yao-Huei, Yang, Pei-Ming, Chuah, Qiu-Yu, Lee, Yi-Jang, Hsieh, Yi-Fen, Peng, Chih-Wen, Chiu, Shu-Jun
Autophagy, 2014-04-30;10(7):1212-28.
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension.
Authors: Sawada H, Saito T, Nickel N, Alastalo T, Glotzbach J, Chan R, Haghighat L, Fuchs G, Januszyk M, Cao A, Lai Y, Perez V, Kim Y, Wang L, Chen P, Spiekerkoetter E, Mitani Y, Gurtner G, Sarnow P, Rabinovitch M
J Exp Med, 2014-01-20;211(2):263-80.
Species: Human
Sample Types: Cell Culture Supernates
-
The evolution of cellular deficiency in GATA2 mutation.
Authors: Dickinson R, Milne P, Jardine L, Zandi S, Swierczek S, McGovern N, Cookson S, Ferozepurwalla Z, Langridge A, Pagan S, Gennery A, Heiskanen-Kosma T, Hamalainen S, Seppanen M, Helbert M, Tholouli E, Gambineri E, Reykdal S, Gottfreethsson M, Thaventhiran J, Morris E, Hirschfield G, Richter A, Jolles S, Bacon C, Hambleton S, Haniffa M, Bryceson Y, Allen C, Prchal J, Dick J, Bigley V, Collin M
Blood, 2013-12-17;123(6):863-74.
Species: Human
Sample Types: Serum
-
Prevention of F-actin assembly switches the response to SCF from chemotaxis to degranulation in human mast cells.
Authors: Smrz D, Bandara G, Beaven M, Metcalfe D, Gilfillan A
Eur J Immunol, 2013-06-04;43(7):1873-82.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased circulation of galectin-3 in cancer induces secretion of metastasis-promoting cytokines from blood vascular endothelium.
Authors: Chen C, Duckworth C, Zhao Q, Pritchard D, Rhodes J, Yu L
Clin Cancer Res, 2013-02-11;19(7):1693-704.
Species: Human
Sample Types: Whole Cells
-
Anti-inflammatory effects of methoxyphenolic compounds on human airway cells.
Authors: Houser KR, Johnson DK, Ishmael FT
J Inflamm (Lond), 2012-03-13;9(0):6.
Species: Human
Sample Types: Cell Culture Supernates
-
p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype.
Authors: Freund A, Patil CK, Campisi J
EMBO J., 2011-03-11;30(8):1536-48.
Species: Human
Sample Types: Cell Culture Supernates
-
The implication of aberrant GM-CSF expression in decidual cells in the pathogenesis of preeclampsia.
Authors: Huang SJ, Zenclussen AC, Chen CP, Basar M, Yang H, Arcuri F, Li M, Kocamaz E, Buchwalder L, Rahman M, Kayisli U, Schatz F, Toti P, Lockwood CJ
Am. J. Pathol., 2010-09-09;177(5):2472-82.
Species: Human
Sample Types: Tissue Homogenates
-
Shaping of iNKT cell repertoire after unrelated cord blood transplantation.
Authors: Beziat V, Nguyen S, Exley M, Achour A, Simon T, Chevallier P, Sirvent A, Vigouroux S, Debre P, Rio B, Vieillard V, Lubrano-Berthelier C, Alatrakchi N
Clin. Immunol., 2010-02-13;135(3):364-73.
Species: Human
Sample Types: Cell Culture Supernates
-
HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial.
Authors: Calmy A, Gayet-Ageron A, Montecucco F, Nguyen A, Mach F, Burger F, Ubolyam S, Carr A, Ruxungtham K, Hirschel B, Ananworanich J
AIDS, 2009-05-15;23(8):929-39.
Species: Human
Sample Types: Plasma
-
HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells.
Authors: Schuster C, Vaculik C, Fiala C, Meindl S, Brandt O, Imhof M, Stingl G, Eppel W, Elbe-Burger A
J. Exp. Med., 2009-01-12;206(1):169-81.
Species: Human
Sample Types: Cell Culture Supernates
-
Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases.
Authors: Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F, Hector A, Eber E, Marcos V, Bittmann I, Eickelberg O, Griese M, Roos D
J. Immunol., 2008-12-01;181(11):8053-67.
Species: Human
Sample Types: BALF
-
Assessment of cervicovaginal cytokine levels following exposure to microbicide Nisin gel in rabbits.
Authors: Aranha CC, Gupta SM, Reddy KV
Cytokine, 2008-05-29;43(1):63-70.
Species: Rabbit
Sample Types: Vaginal Lavage Fluid
-
Isolation and characterization of CD146+ multipotent mesenchymal stromal cells.
Authors: Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, Fatica A, Negrini M, Peschle C, Valtieri M
Exp. Hematol., 2008-05-27;36(8):1035-46.
Species: Human
Sample Types: Cell Culture Supernates
-
Systemic responses after bronchial aspirin challenge in sensitive patients with asthma.
Authors: Makowska JS, Grzegorczyk J, Bienkiewicz B, Wozniak M, Kowalski ML
J. Allergy Clin. Immunol., 2007-12-20;121(2):348-54.
Species: Human
Sample Types: Serum
-
Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts.
Authors: Hatfield K, Ryningen A, Corbascio M, Bruserud O
Int. J. Cancer, 2006-11-15;119(10):2313-21.
Species: Human
Sample Types: Cell Culture Supernates
-
cIAP-2 and survivin contribute to cytokine-mediated delayed eosinophil apoptosis.
Authors: Vassina EM, Yousefi S, Simon D, Zwicky C, Conus S, Simon HU
Eur. J. Immunol., 2006-07-01;36(7):1975-84.
Species: Human
Sample Types: Serum
-
Mast cell beta-tryptase selectively cleaves eotaxin and RANTES and abrogates their eosinophil chemotactic activities.
Authors: Pang L, Nie M, Corbett L, Sutcliffe A, Knox AJ
J. Immunol., 2006-03-15;176(6):3788-95.
Species: Human
Sample Types: Cell Culture Supernates
-
Transmission of Mycobacterium tuberculosis undetected by tuberculin skin testing.
Authors: Anderson ST, Williams AJ, Brown JR, Newton SM, Simsova M, Nicol MP, Sebo P, Levin M, Wilkinson RJ, Wilkinson KA
Am. J. Respir. Crit. Care Med., 2006-02-02;173(9):1038-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Secretory IgA induces antigen-independent eosinophil survival and cytokine production without inducing effector functions.
Authors: Bartemes KR, Cooper KM, Drain KL, Kita H
J. Allergy Clin. Immunol., 2005-10-01;116(4):827-35.
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of GM-CSF release by enantiomers of beta-agonists in human airway smooth muscle.
Authors: Ameredes BT, Calhoun WJ
J. Allergy Clin. Immunol., 2005-07-01;116(1):65-72.
Species: Human
Sample Types: Whole Cells
-
Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells.
Authors: Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC
Cancer Res., 2004-12-15;64(24):9160-6.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of ozone exposure on airway responses to inhaled allergen in asthmatic subjects.
Authors: Chen LL, Tager IB, Peden DB, Christian DL, Ferrando RE, Welch BS, Balmes JR
Chest, 2004-06-01;125(6):2328-35.
Species: Human
Sample Types: BALF
-
Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue.
Authors: Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH
Ann. Neurol., 2004-02-01;55(2):221-35.
Species: Human
Sample Types: CSF
-
Thymus and activation-regulated chemokine (TARC/CCL17) produced by mouse epidermal Langerhans cells is upregulated by TNF-alpha and IL-4 and downregulated by IFN-gamma.
Authors: Xiao T, Fujita H, Saeki H, Mitsui H, Sugaya M, Tada Y, Kakinuma T, Torii H, Nakamura K, Asahina A, Tamaki K
Cytokine, 2003-08-01;23(4):126-32.
Species: Human
Sample Types: Serum
-
Spontaneous circulation of myeloid-lymphoid-initiating cells and SCID-repopulating cells in sickle cell crisis.
Authors: Lamming CE, 175, Augustin L, Blackstad M, Lund TC, Hebbel RP, Verfaillie CM
2003-03-01;111(6):811-9.
Species: Human
Sample Types: Serum
-
Autocrine type I IFN and contact with endothelium promote the presentation of influenza A virus by monocyte-derived APC.
Authors: Qu C, Moran TM, Randolph GJ
J. Immunol., 2003-01-15;170(2):1010-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulation of the interleukin-5 receptor alpha-subunit on peripheral blood eosinophils from healthy subjects.
Authors: Hellman C, 107334, Hallden G, Hylander B, Lundahl J
Lagging strand gap suppression connects BRCA-mediated fork protection to nucleosome assembly through PCNA-dependent CAF-1 recycling, 2003-01-01;131(1):75-81.
Species: Human
Sample Types: Peritoneal Fluid
-
Retroviral interleukin 1alpha gene transfer in bone marrow stromal cells in a primate model: induction of myelopoiesis stimulation.
Authors: de Revel T, Becard N, Sorg T, Rousseau S, Spano JP, Thiebot H, Methali M, Gras G, Le Grand R, Dormont D
Br. J. Haematol., 2002-09-01;118(3):875-84.
Species: Primate - Macaca fascicularis (Crab-eating Monkey or Cynomolgus Macaque)
Sample Types: Cell Culture Supernates
-
Local immunostimulation induced by intravesical administration of autologous interferon-gamma-activated macrophages in patients with superficial bladder cancer.
Authors: Pages F, Lebel-Binay S, Vieillefond A, Deneux L, Cambillau M, Soubrane O, Debre B, Tardy D, Lemonne JL, Abastado JP, Fridman WH, Thiounn N
Clin. Exp. Immunol., 2002-02-01;127(2):303-9.
Species: Human
Sample Types: Urine
-
Distinct hematopoietic support by two human stromal cell lines.
Authors: Loeuillet C, Bernard G, Remy-Martin J, Saas P, Herve P, Douay L, Chalmers D
Exp. Hematol., 2001-06-01;29(6):736-45.
Species: Human
Sample Types: Cell Culture Supernates
-
c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin.
Authors: Szabowski A, Maas-Szabowski N, Andrecht S, Kolbus A, Schorpp-Kistner M, Fusenig NE, Angel P
Cell, 2000-11-22;103(5):745-55.
Species: Human
Sample Types: Cell Culture Supernates
-
Bleomycin stimulates lung fibroblast and epithelial cell lines to release eosinophil chemotactic activity.
Authors: Sato E, Koyama S
Eur. Respir. J., 2000-11-01;16(5):951-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Natural interferon alpha/beta-producing cells link innate and adaptive immunity.
Authors: Kadowaki N, Antonenko S, Lau JY, Liu YJ
J. Exp. Med., 2000-07-17;192(2):219-26.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum levels of interleukins, growth factors and angiogenin in patients with endometrial cancer.
Authors: Chopra V, Dinh TV, Hannigan EV
J. Cancer Res. Clin. Oncol., 1997-01-01;123(3):167-72.
Species: Human
Sample Types: Serum
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human GM-CSF Quantikine ELISA Kit
Average Rating: 5 (Based on 4 Reviews)
Have you used Human GM-CSF Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Clear instructions and robust method