Human HB-EGF Antibody Summary
rhHRG-beta, rhEGF, or rhTGF-alpha.
Asp63-Leu148
Accession # Q53H93
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
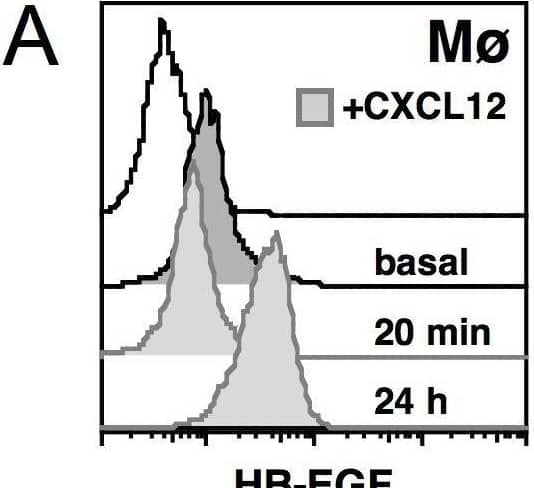
Detection of HB-EGF by Flow Cytometry CXCL12 modifies HB-EGF expression in mononuclear phagocytes. Human mononuclear phagocytes (Mø) were cultured alone or in the presence of 200 ng/mL CXCL12. Cells were collected after 20 minutes, 2 hours and 24 hours; cell-free supernatants were collected after 24 hours and the levels of soluble HB-EGF protein were measured using a specific ELISA. (A) Flow cytometric analysis showing that CXCL12-stimulated Mø released HB-EGF (after 20 minutes) and up-regulated its expression (after 24 hours). (B) Northern blot analysis on Mø and neutrophils (PMN, used as negative control) collected after 2 hours of stimulation with CXCL12. Only Mø produced detectable levels of HB-EGF mRNA in basal conditions, and HB-EGF transcripts were up-regulated upon stimulation. After 24 hours, the mRNA up-regulation persisted (data not shown). (C) CXCL12 treatment induced Mø to release HB-EGF into the culture medium (p < 0.05). Representative pictures or the means ± SD out of 10 experiments are shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/20946648), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
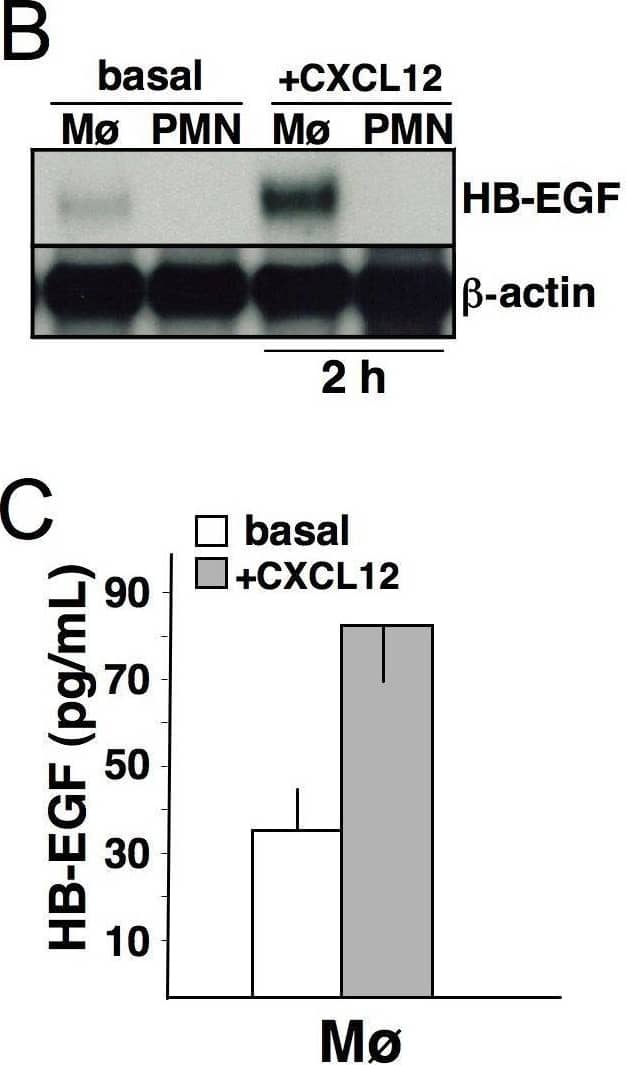
Detection of HB-EGF by Western Blot CXCL12 modifies HB-EGF expression in mononuclear phagocytes. Human mononuclear phagocytes (Mø) were cultured alone or in the presence of 200 ng/mL CXCL12. Cells were collected after 20 minutes, 2 hours and 24 hours; cell-free supernatants were collected after 24 hours and the levels of soluble HB-EGF protein were measured using a specific ELISA. (A) Flow cytometric analysis showing that CXCL12-stimulated Mø released HB-EGF (after 20 minutes) and up-regulated its expression (after 24 hours). (B) Northern blot analysis on Mø and neutrophils (PMN, used as negative control) collected after 2 hours of stimulation with CXCL12. Only Mø produced detectable levels of HB-EGF mRNA in basal conditions, and HB-EGF transcripts were up-regulated upon stimulation. After 24 hours, the mRNA up-regulation persisted (data not shown). (C) CXCL12 treatment induced Mø to release HB-EGF into the culture medium (p < 0.05). Representative pictures or the means ± SD out of 10 experiments are shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/20946648), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
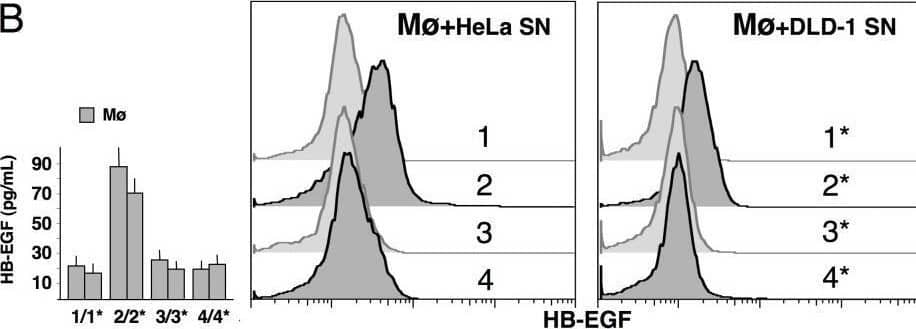
Detection of HB-EGF by Flow Cytometry Knockdown of GM-CSF protein levels after siRNA application in cancer cells. HeLa/DLD-1 cells were transfected with control siRNA (1/1*, 2/2*) or GM-CSF siRNA (3/3*, 4/4*) and cultured in the absence or presence of 25 ng/mL HB-EGF. The numbers indicate the culture conditions and the corresponding supernatants (SN) used for ELISA or cell stimulation. (A) Blockade of GM-CSF production in cultures of HeLa/DLD-1 cells transfected with GM-CSF siRNA was confirmed by immunocytochemistry (2/2* vs. 4/4*) and ELISA (left side; 2/2* vs. 4/4*, p < 0.05). (B) SN from GM-CSF-silenced HeLa/DLD-1 did not induce HB-EGF expression in mononuclear phagocytes (Mø), as revealed by flow cytometry (2/2* vs. 4/4*) and ELISA (left side; 2/2* vs. 4/4*, p < 0.05). (C) Mø stimulated with SN from GM-CSF-silenced HeLa/DLD-1 cells released SN less effective at inducing GM-CSF in non-silenced cancer cells, as determined by ELISA (see Methods section; SN2 vs. SN4, p < 0.05). Representative pictures or the means ± SD out of 5 experiments are shown. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/20946648), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: HB-EGF
HB-EGF was originally purified based on its heparin-binding property and mitogenic activity on BALB-3T3 fibroblasts from the conditioned medium of the human U-937 histiocytic lymphoma cell line. The natural protein has an apparent molecular mass of 19-23 kDa and exists in multiple forms as a result of heterogenous
O‑glycosylation and/or N-terminal truncation. In addition to fibroblasts, HB-EGF is also a potent mitogen for keratinocytes and smooth muscle cells but not for capillary endothelial cells. HB-EGF is produced in monocytes and macrophages. In addition, transcription of HB-EGF can be induced in vascular endothelial cells as well as aortic smooth muscle cells (SMC), suggesting that HB-EGF may have an important role in the pathogenesis of atherosclerosis.
HB-EGF is a member of the EGF family of mitogens which also include transforming growth factor-alpha (TGF-alpha ), amphiregulin (AR), rat schwanoma-derived growth factor (SDGF), vaccinia growth factor (VGF), and the various ligands for the HER2/ErbB2/Neu receptor. All these cytokines are derived from transmembrane precursors that contain one or several EGF structural units in their extracellular domain. Many of these transmembrane precursors are biologically active and seem to play a role in juxtacrine stimulation of adjacent cells. The cDNA for HB-EGF encodes a 204 amino acid residue transmembrane protein that is proteolytically cleaved to generate the soluble HB-EGF. Like EGF, TGF-alpha, and AR; HB-EGF binds to the EGF receptor and activates the receptor tyrosine kinase. HB-EGF is reported to be a more potent SMC mitogen than EGF. It has been suggested that the differential activities found for HB-EGF compared to EGF may be mediated by the
heparin-binding properties of HB-EGF. A diphtheria toxin receptor that mediates the endocytosis of the bound toxin has been cloned and found to be identical to the transmembrane HB-EGF precursor.
Product Datasheets
Citations for Human HB-EGF Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
2
Citations: Showing 1 - 2
Filter your results:
Filter by:
-
Amphiregulin Exosomes Increase Cancer Cell Invasion
Authors: James N. Higginbotham, Michelle Demory Demory Beckler, Jonathan D. Gephart, Jeffrey L. Franklin, Galina Bogatcheva, Gert-Jan Kremers et al.
Current Biology
-
The response of human epithelial cells to TNF involves an inducible autocrine cascade.
Authors: Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK
Cell, 2006-03-24;124(6):1225-39.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human HB-EGF Antibody
There are currently no reviews for this product. Be the first to review Human HB-EGF Antibody and earn rewards!
Have you used Human HB-EGF Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image

