Ko 143
Tocris Bioscience | Catalog # 3241

Key Product Details
Description
Product Description
Ko 143 is a potent and selective breast cancer resistance protein multidrug transporter (BCRP) inhibitor (EC90 = 26 nM). Displays > 200-fold selectivity over P-gp and MRP-1 transporters. Increases intracellular drug accumulation and reverses BCRP-mediated multidrug resistance. Inhibits ABCB1 and ABCC1 at higher concentrations. Rapidly metabolized in rat plasma.
Product Specifications for Ko 143
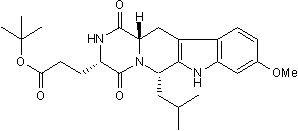
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 23.48 | 50 | |
| Ethanol | 46.96 | 100 |
Preparing Stock Solutions for Ko 143
The following data is based on the product molecular weight 469.57.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 1 mM | 2.13 mL | 10.65 mL | 21.30 mL |
| 5 mM | 0.43 mL | 2.13 mL | 4.26 mL |
| 10 mM | 0.21 mL | 1.06 mL | 2.13 mL |
| 50 mM | 0.04 mL | 0.21 mL | 0.43 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 105 publications citing the usage of this product.
- Weidner The inhibitor Ko143 is not specific for ABCG2. J.Pharmacol.Exp.Ther. 2015 PMID: 26148857
- Allen Mouse breast cancer resistance protein (Bcrp1/Abcg2) mediates etop. resistance and transport, but etop. oral availability is limited primarily by P-glycoprotein. Cancer Res. 2003 PMID: 12649196
- Loevezijn Inhibition of BCRP-mediated drug efflux by fumitremorgin-type indolyl diketopiperazines. Bioorg.Med.Chem.Letts. 2001 PMID: 11140726
- Allen Potent and specific inhibition of breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol.Cancer Ther. 2002 PMID: 12477054
Product Documents for Ko 143
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Ko 143
For research use only
Citations for Ko 143
Customer Reviews for Ko 143
There are currently no reviews for this product. Be the first to review Ko 143 and earn rewards!
Have you used Ko 143?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
