Mouse CCL17/TARC Quantikine ELISA Kit Summary
Product Summary
Precision
Cell Culture Supernates, Serum, EDTA Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 27 | 89 | 278 | 29 | 98 | 310 |
| Standard Deviation | 1.3 | 2.3 | 7.8 | 2.2 | 6.9 | 17.3 |
| CV% | 4.8 | 2.6 | 2.8 | 7.6 | 7 | 5.6 |
Recovery
The recovery of mouse TARC spiked to three levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Supernates (n=6) | 94 | 85-113 |
| EDTA Plasma (n=6) | 94 | 83-109 |
| Serum (n=6) | 94 | 82-103 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: CCL17/TARC
CCL17/TARC is a chemokine that interacts with CCR4 and CCR8 to induce the chemoattraction of activated Th2 cells, basophils, and NK cells. It is produced by thymic dendritic cells, lymph node dendritic cells, monocytes, CD4+ T cells, keratinocytes, fibroblasts, bronchial epithelial cells, and Reed-Sternberg cells. CCL17 promotes Th2 cell recruitment to sites of inflammation and induces platelet aggregation and degranulation.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 50 µL of Assay Diluent to each well.
- Add 50 µL of Standard, Control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process 4 times for a total of 5 washes.
- Add 100 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours.
- Aspirate and wash 5 times.
- Add 100 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 100 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Mouse CCL17/TARC Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
45
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Matrine regulates Th1/Th2 inflammatory responses by inhibiting the Hsp90/NF-kappaB signaling axis to alleviate atopic dermatitis
Authors: P Huang, F Hu, ZB Yang, Y Pan, R Zhou, YN Yan, HZ Wang, C Wang
The Kaohsiung journal of medical sciences, 2023-02-09;0(0):.
Species: Mouse
Sample Types: Serum
-
Type I interferon antagonism of the JMJD3-IRF4 pathway modulates macrophage activation and polarization
Authors: K Ming-Chin, AA Achuthan, DP De Souza, TJ Lupancu, KJ Binger, MKS Lee, Y Xu, MJ McConville, NA de Weerd, D Dragoljevi, PJ Hertzog, AJ Murphy, JA Hamilton, AJ Fleetwood
Cell Reports, 2022-04-19;39(3):110719.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Chronic Allergen Challenge Induces Corticosteroid Insensitivity With Persistent Airway Remodeling and Type 2 Inflammation
Authors: BW Lewis, ML Ford, AQ Khan, J Walum, RD Britt
Frontiers in Pharmacology, 2022-04-11;13(0):855247.
Species: Mouse
Sample Types: BALF
-
Anti-fibrotic effect of Pycnogenol� in a polyhexamethylene guanidine-treated mouse model
Authors: CM Park, HY Kim, D Jeon, YJ Shin, IH Kim, SJ Choi, KC Kim, K Lee, SH Kim, MS Kim
Respiratory physiology & neurobiology, 2021-10-12;0(0):103802.
Species: Mouse
Sample Types: Tissue Homogenates
-
Isoalantolactone alleviates ovalbumin?induced asthmatic inflammation by reducing alternatively activated macrophage and STAT6/PPAR?gamma/KLF4 signals
Authors: Y Song, X Li, F Liu, H Zhu, Y Shen
Molecular Medicine Reports, 2021-08-09;24(4):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Neutralization of IL-33 modifies the type 2 and type 3 inflammatory signature of viral induced asthma exacerbation
Authors: KJ Warren, JA Poole, JM Sweeter, JM DeVasure, JD Dickinson, RS Peebles, TA Wyatt
Respiratory Research, 2021-07-15;22(1):206.
Species: Mouse
Sample Types: BALF
-
A New Mouse Model of Chronic Myocarditis Induced by Recombinant Bacille Calmette-Gu�rin Expressing a T-Cell Epitope of Cardiac Myosin Heavy Chain-&alpha
Authors: K Tajiri, K Imanaka-Yo, Y Tsujimura, K Matsuo, M Hiroe, K Aonuma, M Ieda, Y Yasutomi
International Journal of Molecular Sciences, 2021-01-14;22(2):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Combretum quadrangulare Extract Attenuates Atopic Dermatitis-Like Skin Lesions through Modulation of MAPK Signaling in BALB/c Mice
Authors: JH Park, MH Hwang, YR Cho, SS Hong, JS Kang, WH Kim, SH Yang, DW Seo, JS Oh, EK Ahn
Molecules, 2020-04-24;25(8):.
Species: Mouse
Sample Types: Serum
-
Intratumoral IFN-? gene delivery reduces tumor-infiltrating regulatory T cells through the downregulation of tumor CCL17 expression
Authors: A Hirata, H Hashimoto, C Shibasaki, K Narumi, K Aoki
Cancer Gene Ther., 2018-11-12;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Eosinophil recruitment is dynamically regulated by interplay among lung dendritic cell subsets after allergen challenge
Authors: S Yi, J Zhai, R Niu, G Zhu, M Wang, J Liu, H Huang, Y Wang, X Jing, L Kang, W Song, Y Shi, H Tang
Nat Commun, 2018-09-24;9(1):3879.
Species: Mouse
Sample Types: BALF
-
Sox7 promotes high-grade glioma by increasing VEGFR2-mediated vascular abnormality
Authors: IK Kim, K Kim, E Lee, DS Oh, CS Park, S Park, JM Yang, JH Kim, HS Kim, DT Shima, JH Kim, SH Hong, YH Cho, YH Kim, JB Park, GY Koh, YS Ju, HK Lee, S Lee, I Kim
J. Exp. Med., 2018-02-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
Airway inflammation after epicutaneous sensitization of mice requires protease activity of low-dose allergen inhalation
Authors: I Nishioka, T Takai, N Maruyama, S Kamijo, P Suchiva, M Suzuki, S Kunimine, H Ochi, S Shimura, K Sudo, H Ogawa, K Okumura, S Ikeda
J. Allergy Clin. Immunol., 2018-02-06;0(0):.
Species: Mouse
Sample Types: BALF
-
Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis.
Authors: Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan D, Kong H, Amagai M, Nagao K
Immunity, 2015-04-21;42(4):756-66.
Species: Mouse
Sample Types: Serum
-
Dendritic cells induce Th2-mediated airway inflammatory responses to house dust mite via DNA-dependent protein kinase.
Authors: Mishra, Amarjit, Brown, Alexandr, Yao, Xianglan, Yang, Shutong, Park, Sung-Jun, Liu, Chengyu, Dagur, Pradeep, McCoy, J Philip, Keeran, Karen J, Nugent, Gayle Z, Jeffries, Kenneth, Qu, Xuan, Yu, Zu-Xi, Levine, Stewart, Chung, Jay H
Nat Commun, 2015-02-18;6(0):6224.
Species: Mouse
Sample Types: BALF
-
Mycoplasma pneumoniae CARDS toxin exacerbates ovalbumin-induced asthma-like inflammation in BALB/c mice.
Authors: Medina, Jorge L, Coalson, Jacqueli, Brooks, Edward G, Le Saux, Claude J, Winter, Vicki T, Chaparro, Adriana, Principe, Molly F, Solis, Laura, Kannan, T R, Baseman, Joel B, Dube, Peter H
PLoS ONE, 2014-07-24;9(7):e102613.
Species: Mouse
Sample Types: BALF
-
Cleavage of the T cell protein tyrosine phosphatase by the hepatitis C virus nonstructural 3/4A protease induces a Th1 to Th2 shift reversible by ribavirin therapy.
Authors: Brenndorfer E, Brass A, Karthe J, Ahlen G, Bode J, Sallberg M
J Immunol, 2014-01-17;192(4):1671-80.
Species: Mouse
Sample Types: Cell Lysates
-
Hypomorphic mutation in the RAG2 gene affects dendritic cell distribution and migration.
Authors: Maina, Virginia, Marrella, Veronica, Mantero, Stefano, Cassani, Barbara, Fontana, Elena, Anselmo, Achille, Del Prete, Annalisa, Sozzani, Silvano, Vezzoni, Paolo, Poliani, Pietro L, Villa, Anna
J Leukoc Biol, 2013-09-19;94(6):1221-30.
Species: Mouse
Sample Types: Tissue Homogenates
-
Chitin elicits CCL2 from airway epithelial cells and induces CCR2-dependent innate allergic inflammation in the lung.
J. Immunol., 2012-07-30;189(5):2545-52.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-22 Is Produced by Innate Lymphoid Cells and Limits Inflammation in Allergic Airway Disease.
Authors: Taube C, Tertilt C, Gyulveszi G, Dehzad N, Kreymborg K, Schneeweiss K, Michel E, Reuter S, Renauld JC, Arnold-Schild D, Schild H, Buhl R, Becher B
PLoS ONE, 2011-07-18;6(7):e21799.
Species: Mouse
Sample Types: BALF
-
Reciprocal expression of IL-25 and IL-17A is important for allergic airways hyperreactivity.
Authors: Barlow JL, Flynn RJ, Ballantyne SJ, McKenzie AN
Clin. Exp. Allergy, 2011-07-04;41(10):1447-55.
Species: Mouse
Sample Types: BALF
-
Thymic stromal lymphopoietin is produced by dendritic cells.
Authors: Kashyap M, Rochman Y, Spolski R
J. Immunol., 2011-06-20;187(3):1207-11.
Species: Mouse
Sample Types: BALF
-
IL-33-activated dendritic cells are critical for allergic airway inflammation.
Authors: Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B
Eur. J. Immunol., 2011-05-25;41(6):1675-86.
Species: Mouse
Sample Types: BALF
-
Dual functions of prostaglandin D2 in murine contact hypersensitivity via DP and CRTH2.
Authors: Yamamoto Y, Otani S, Hirai H, Nagata K, Aritake K, Urade Y, Narumiya S, Yokozeki H, Nakamura M, Satoh T
Am. J. Pathol., 2011-05-10;179(1):302-14.
Species: Mouse
Sample Types: Tissue Homogenates
-
Thymic stromal lymphopoietin is a key mediator of breast cancer progression.
Authors: Olkhanud PB, Rochman Y, Bodogai M
J. Immunol., 2011-04-13;186(10):5656-62.
Species: Mouse
Sample Types: Plasma
-
Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation.
Authors: Louten J, Rankin AL, Li Y
Int. Immunol., 2011-03-21;23(5):307-15.
Species: Mouse
Sample Types: BALF
-
IL-33 and M2a alveolar macrophages promote lung defense against the atypical fungal pathogen Pneumocystis murina.
Authors: Nelson MP, Christmann BS, Werner JL, Metz AE, Trevor JL, Lowell CA, Steele C
J. Immunol., 2011-01-10;186(4):2372-81.
Species: Mouse
Sample Types: Tissue Homogenates
-
Foxa2 programs Th2 cell-mediated innate immunity in the developing lung.
Authors: Chen G, Wan H, Luo F
J. Immunol., 2010-05-05;184(11):6133-41.
Species: Mouse
Sample Types: BALF
-
Murine atopic dermatitis responds to peroxisome proliferator-activated receptors alpha and beta/delta (but not gamma) and liver X receptor activators.
Authors: Hatano Y, Man MQ, Uchida Y, Crumrine D, Mauro TM, Feingold KR, Elias PM, Holleran WM
J. Allergy Clin. Immunol., 2009-10-08;125(1):160-9.e1-5.
Species: Mouse
Sample Types: Serum
-
Lovastatin inhibits antigen-induced airway eosinophilia without affecting the production of inflammatory mediators in mice.
Authors: Chiba Y, Sato S, Misawa M
Inflamm. Res., 2009-05-06;0(0):.
Species: Mouse
Sample Types: BALF
-
Disruption of interleukin-1 signaling improves the quality of wound healing.
Authors: Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW, Reichner JS, Albina JE
Am. J. Pathol., 2009-04-23;174(6):2129-36.
Species: Mouse
Sample Types: Tissue Secretion
-
Maintenance of an acidic stratum corneum prevents emergence of murine atopic dermatitis.
Authors: Hatano Y, Man MQ, Uchida Y, Crumrine D, Scharschmidt TC, Kim EG, Mauro TM, Feingold KR, Elias PM, Holleran WM
J. Invest. Dermatol., 2009-01-29;129(7):1824-35.
Species: Mouse
Sample Types: Serum
-
CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation.
Authors: Medoff BD, Seung E, Hong S, Thomas SY, Sandall BP, Duffield JS, Kuperman DA, Erle DJ, Luster AD
J. Immunol., 2009-01-01;182(1):623-35.
Species: Mouse
Sample Types: BALF
-
Cyclooxygenase inhibition during allergic sensitization increases STAT6-independent primary and memory Th2 responses.
Authors: Zhou W, Newcomb DC, Moore ML, Goleniewska K, O'Neal JF, Peebles RS
J. Immunol., 2008-10-15;181(8):5360-7.
Species: Mouse
Sample Types: Tissue Homogenates
-
An eosinophil immune response characterizes the inflammatory skin disease observed in Tie-2 transgenic mice.
Authors: Voskas D, Babichev Y, Ling LS, Alami J, Shaked Y, Kerbel RS, Ciruna B, Dumont DJ
J. Leukoc. Biol., 2008-05-01;84(1):59-67.
Species: Mouse
Sample Types: Serum
-
A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis.
Authors: Trujillo G, O'Connor EC, Kunkel SL, Hogaboam CM
Am. J. Pathol., 2008-04-10;172(5):1209-21.
Species: Mouse
Sample Types: Tissue Homogenates
-
Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells.
Authors: Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ
J. Exp. Med., 2008-03-03;205(3):699-710.
Species: Mouse
Sample Types: BALF
-
iNKT cells require CCR4 to localize to the airways and to induce airway hyperreactivity.
Authors: Meyer EH, Wurbel MA, Staton TL, Pichavant M, Kan MJ, Savage PB, DeKruyff RH, Butcher EC, Campbell JJ, Umetsu DT
J. Immunol., 2007-10-01;179(7):4661-71.
Species: Mouse
Sample Types: Tissue Homogenates
-
The direct action of 1alpha,25(OH)2-vitamin D3 on purified mouse Langerhans cells.
Authors: Fujita H, Asahina A, Komine M, Tamaki K
Cell. Immunol., 2007-05-15;245(2):70-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Airway epithelial STAT3 is required for allergic inflammation in a murine model of asthma.
Authors: Simeone-Penney MC, Severgnini M, Tu P, Homer RJ, Mariani TJ, Cohn L, Simon AR
J. Immunol., 2007-05-15;178(10):6191-9.
Species: Mouse
Sample Types: BALF
-
Therapeutic targeting of CC ligand 21 or CC chemokine receptor 7 abrogates pulmonary fibrosis induced by the adoptive transfer of human pulmonary fibroblasts to immunodeficient mice.
Authors: Pierce EM, Carpenter K, Jakubzick C, Kunkel SL, Flaherty KR, Martinez FJ, Hogaboam CM
Am. J. Pathol., 2007-04-01;170(4):1152-64.
Species: Mouse
Sample Types: Tissue Homogenates
-
Prolonged inhaled allergen exposure can induce persistent tolerance.
Authors: Van Hove CL, Maes T, Joos GF, Tournoy KG
Am. J. Respir. Cell Mol. Biol., 2007-01-11;36(5):573-84.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Local production of chemokines and prostaglandin E2 in the acute, chronic and recovery phase of murine experimental colitis.
Authors: Drmotova M, Rehnstrom E, Jansson L
Cytokine, 2006-11-07;35(5):275-83.
Species: Mouse
Sample Types: Tissue Homogenates
-
CCL17 transgenic mice show an enhanced Th2-type response to both allergic and non-allergic stimuli.
Authors: Tsunemi Y, Saeki H, Nakamura K, Nagakubo D, Nakayama T, Yoshie O, Kagami S, Shimazu K, Kadono T, Sugaya M, Komine M, Matsushima K, Tamaki K
Eur. J. Immunol., 2006-08-01;36(8):2116-27.
Species: Mouse
Sample Types: Serum
-
Immunosuppressant triptolide inhibits dendritic cell-mediated chemoattraction of neutrophils and T cells through inhibiting Stat3 phosphorylation and NF-kappaB activation.
Authors: Liu Q, Chen T, Chen G, Li N, Wang J, Ma P, Cao X
Biochem. Biophys. Res. Commun., 2006-05-12;345(3):1122-30.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inhalation tolerance is induced selectively in thoracic lymph nodes but executed pervasively at distant mucosal and nonmucosal tissues.
Authors: Alvarez D, Swirski FK, Yang TC, Fattouh R, Croitoru K, Bramson JL, Stampfli MR, Jordana M
J. Immunol., 2006-02-15;176(4):2568-80.
Species: Mouse
Sample Types: Serum
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse CCL17/TARC Quantikine ELISA Kit
Average Rating: 5 (Based on 2 Reviews)
Have you used Mouse CCL17/TARC Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
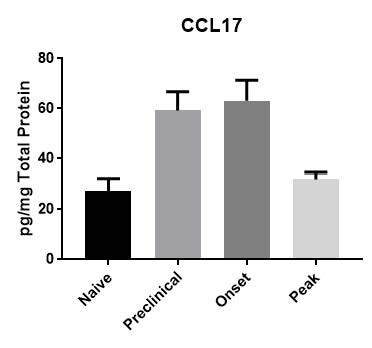
CCL17 detected following cytokine stimulation