Okadaic acid
Tocris Bioscience | Catalog # 1136

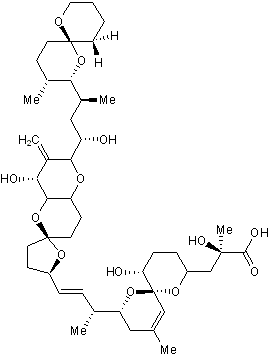
Key Product Details
Description
Product Description
Okadaic acid is a potent inhibitor of protein phosphatase 1 (IC50 = 3 nM) and protein phosphatase 2A (IC50 = 0.2-1 nM). Displays > 100,000,000-fold selectivity over PP2B and PP2C. Tumor promoter. Shown to activate atypical protein kinase C in adipocytes.
Okadaic acid ammonium salt (Cat. No. 9012) also available.
Product Specifications for Okadaic acid
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| DMSO | 40 | 50 |
Preparing Stock Solutions for Okadaic acid
The following data is based on the product molecular weight 805.01.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 0.5 mM | 2.48 mL | 12.42 mL | 24.84 mL |
| 2.5 mM | 0.50 mL | 2.48 mL | 4.97 mL |
| 5 mM | 0.25 mL | 1.24 mL | 2.48 mL |
| 25 mM | 0.05 mL | 0.25 mL | 0.50 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 132 publications citing the usage of this product.
- Standaert Okadaic acid activates atypical protein kinase C (ζ/λ) in rat and 3T3/L1 adipocytes. J.Biol.Chem. 1999 PMID: 10318822
- Nuydens Okadaic acid-induced apoptosis in neuronal cells: evidence for an abortive mitotic attempt. J.Neurochem. 1998 PMID: 9489733
- Haystead Effects of the tumour promotor okadaic acid on intracellular protein phosphorylation and metabolism. Nature 1989 PMID: 2562908
- McCluskey Serine-threonine protein phosphatase inhibitors: development of therapeutic strategies. J.Med.Chem. 2002 PMID: 11881984
- Cohen Okadaic acid: a new probe for the study of cellular regulation. TiBS 1990 PMID: 2158158
Product Documents for Okadaic acid
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Okadaic acid
For research use only
Related Research Areas
Citations for Okadaic acid
Customer Reviews for Okadaic acid (2)
Have you used Okadaic acid?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Species: RatAssay Type: In VitroCell Line/Tissue: Sprague-Dawley rats primary neuronalVerified Customer | Posted 04/04/2020Okadaic acid for PP2A at 20 nM
-
Species: MouseAssay Type: In VitroCell Line/Tissue: CardiomyocytesVerified Customer | Posted 05/15/2019HL-1 cardiomyocytes were treated with 100 nM fostriecin, 100 nM Okadaic acid or 50 nM 8-bromo-cAMP for 30 min followed by Western blot analysis for pCaMKII expression. Okadaic acid significantly increased phosphorylation of CaMKII; indicating a major role of PP1 in CaMKII dephosphorylation.
There are no reviews that match your criteria.


