Recombinant Human Thrombopoietin/TPO GMP Protein, CF
Recombinant Human Thrombopoietin/TPO GMP Protein, CF Summary
- TPO Manufactured in Bio-Techne's new GMP facility
- Lot-to-lot consistency
- Stringent guidelines for patient safety
- Scalability necessary to support successful therapeutics
- Learn more about manufacturing in our new GMP facility
- Test it in your process! Request a sample of GMP TPO
Product Specifications
Ser22 - Leu195 with an N-terminal Alanine
Produced using non-animal reagents in an animal-free laboratory.
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
BT-TPO-GMP
| Formulation | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in sterile water. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
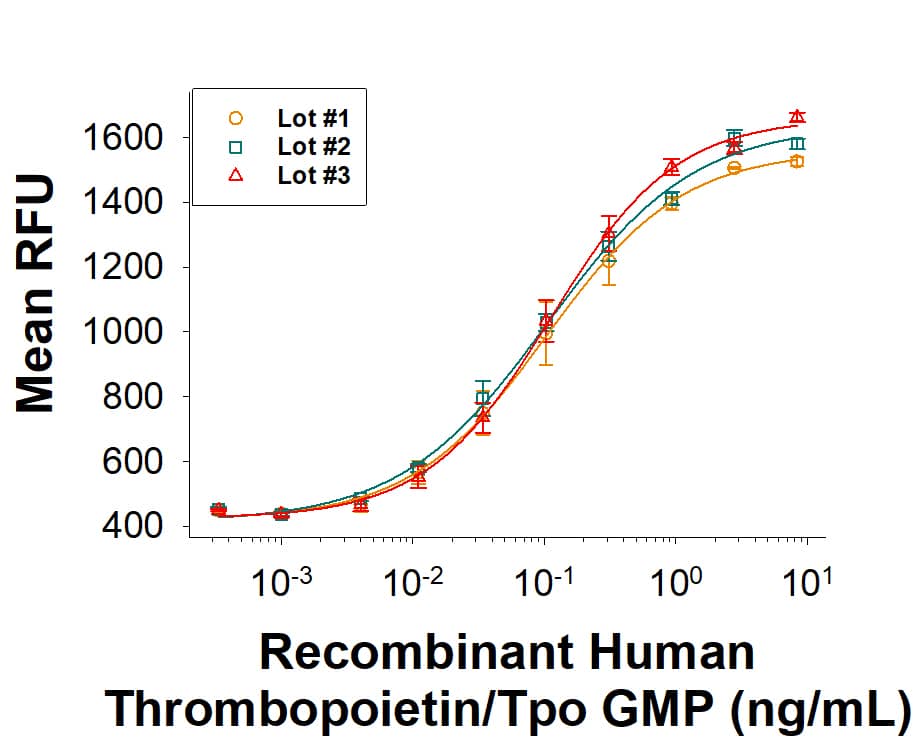
The bioactivity of Recombinant Human Thrombopoietin/TPO GMP Protein (Catalog # BT-TPO-GMP) was measured in a cell proliferation assay using MO7e human megakaryocytic leukemic cells. The ED50 for this effect is 0.0500‑0.500 ng/mL. Three independent lots were tested for bioactivity and plotted on the same graph to show lot-to-lot consistency of GMP Thrombopoietin/Tpo protein.
 View Larger
View Larger
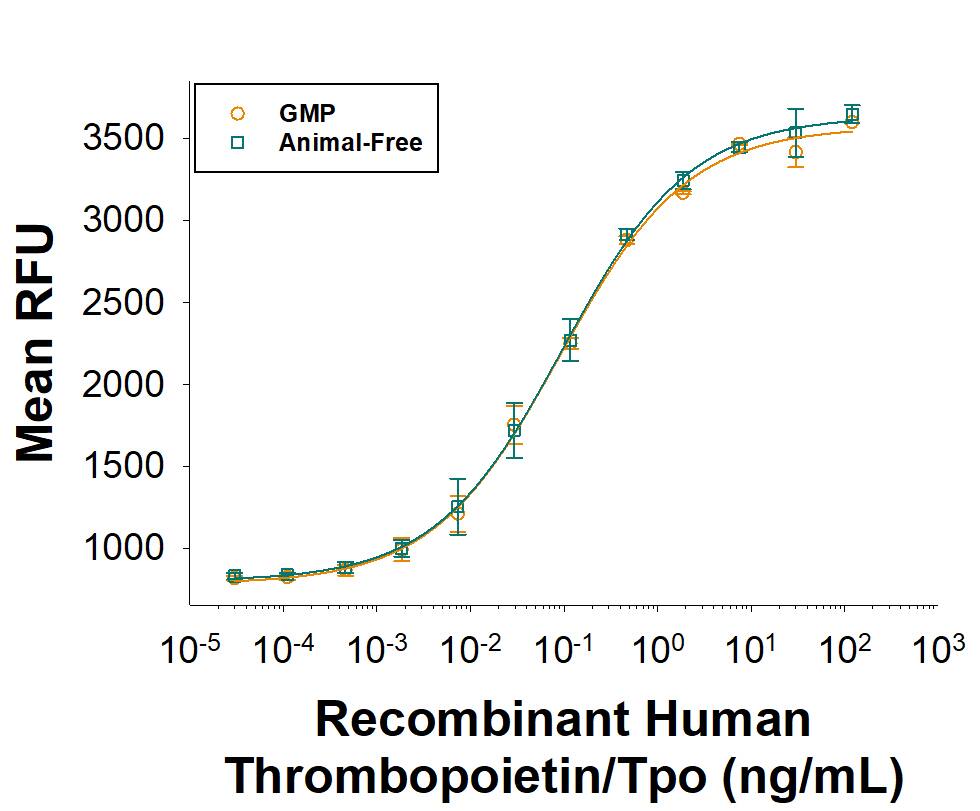
Equivalent bioactivity of GMP (Catalog # BT-TPO-GMP) and Animal-Free (BT-TPO-AFL) grades of Recombinant Human Thrombopoietin/TPO as measured in a cell proliferation assay using MO7e human megakaryocytic leukemic cells (orange and green, respectively).
 View Larger
View Larger
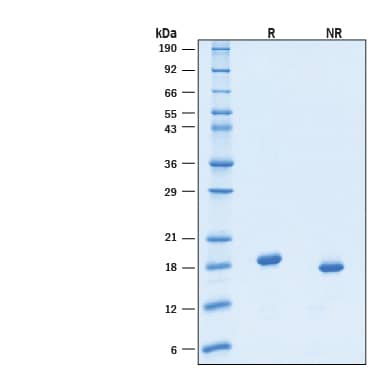
2 μg/lane of Recombinant Human Thrombopoietin/TPO GMP Protein (Catalog # BT-TPO-GMP) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 18-19 kDa under reducing conditions.
 View Larger
View Larger
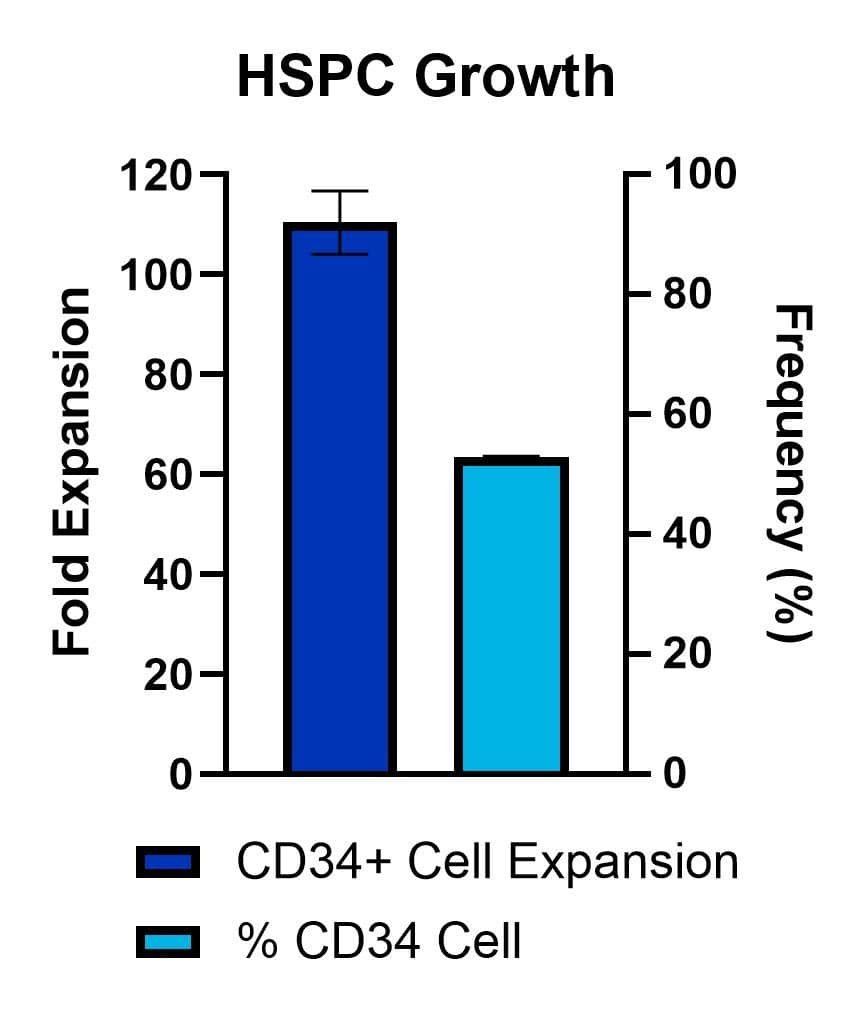
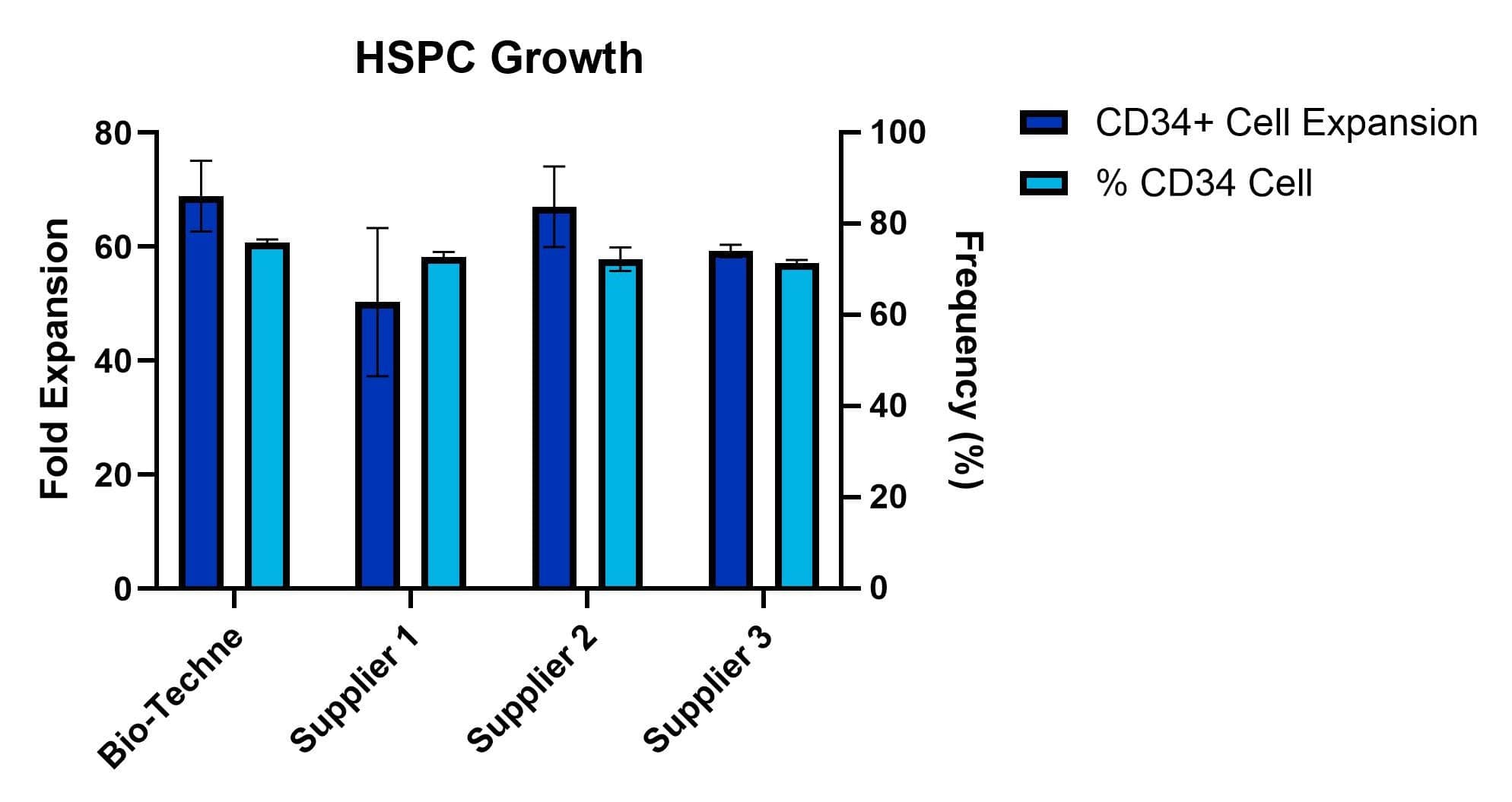
Purified CD34+ cells derived from plerixafor mobilized peripheral blood were cultured in commercially available media. The media was supplemented with SCF (100 ng/mL, Catalog # BT-SCF-GMP), TPO (100 ng/mL, Catalog # BT-TPO-GMP), FLT-3L (100 ng/mL, Catalog # BT-FT3L-GMP), and SR1 (1 µM, Catalog # 7086). After 7 days, fold expansion was calculated from total viable cell counts. Frequency (%) CD34+ cells was analyzed by flow cytometry on Days 0 and 7. The average fold expansion of n=2 technical replicates is shown.
 View Larger
View Larger
Purified CD34+ cells derived from plerixafor mobilized peripheral blood were cultured in commercially available media. The media was supplemented with SCF (Catalog # BT-SCF-GMP, 100 ng/mL), TPO (Catalog # BT-TPO-GMP, 100 ng/mL), FLT-3L (Catalog # BT-FT3L-GMP, 100 ng/mL), and SR1 (Catalog # 7086, 1 µM). After 7 days, fold expansion was calculated from total viable cell counts. Percent (%) CD34+ cells was analyzed by flow cytometry on Days 0 and 7. The average fold expansion of n=3 technical replicates is shown.
Reconstitution Calculator
Background: Thrombopoietin/Tpo
Thrombopoietin (TPO) is a crucial regulator of hematopoietic stem cell (HSC) differentiation, maturation, and proliferation, as well as megakaryocytopoiesis and thrombopoiesis. TPO is often used in conjunction with Stem Cell Factor (SCF) and Flt-3 ligand in cell culture protocols to expand HSCs for bone marrow transplantation and cellular therapies. When combined with SCF and Flt-3 ligand, it promotes the differentiation of HSCs into megakaryocytes, leading to the production of platelets. It has shown promise in emerging cellular therapies for treating sickle cell disease, beta thalassemia, and other blood-related disorders. Moreover, TPO in combination with other cytokines is utilized in the generation of iNK cells, which have the potential to be incorporated into cellular immunotherapies.
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and Eu. Ph. 5.2.12, Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products.
R&D Systems' quality focus includes:
- Designed, manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented and controlled manufacturing process
- Control of documentation and process changes by QA
- Personnel training programs
- Raw material inspection and vendor qualification/monitoring program
- Validated equipment, processes and test methods
- Equipment calibration and maintenance schedules using a Regulatory Asset Manager
- Facility/Utilities maintenance, contamination controls, safety and pest control programs
- Material review process for variances
- Robust product stability program following relevant ICH guidelines
- N-terminal amino acid analysis
- SDS-PAGE purity analysis
- Molecular weight analysis via mass spectrometry
- Endotoxin assessment per USP <85> and Ph. Eur. 2.6.14 guidelines
- Bioassay analysis
- Microbial testing per USP <71> and Ph. Eur. 2.6.1 guidelines
- Host cell protein assessment
- Host cell DNA assessment
- Mycoplasma assessment
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis and St. Paul, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers.
Product Specific Notices
Full terms and conditions of sale can be found online in the Protein Sciences Segment T&Cs at: Terms & Conditions.FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human Thrombopoietin/TPO GMP Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant Human Thrombopoietin/TPO GMP Protein, CF and earn rewards!
Have you used Recombinant Human Thrombopoietin/TPO GMP Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image



