Recombinant Mouse IL-23 Protein
Recombinant Mouse IL-23 Protein Summary
Product Specifications
| Mouse IL-23 p40 (Met23-Ser335) Accession # P43432 |
IGSGSSRGGSGSGGSGGGGSK | Mouse IL-23 p19 (Leu20-Ala196) Accession # Q9EQ14 |
| N-terminus | C-terminus | |
Analysis
Product Datasheets
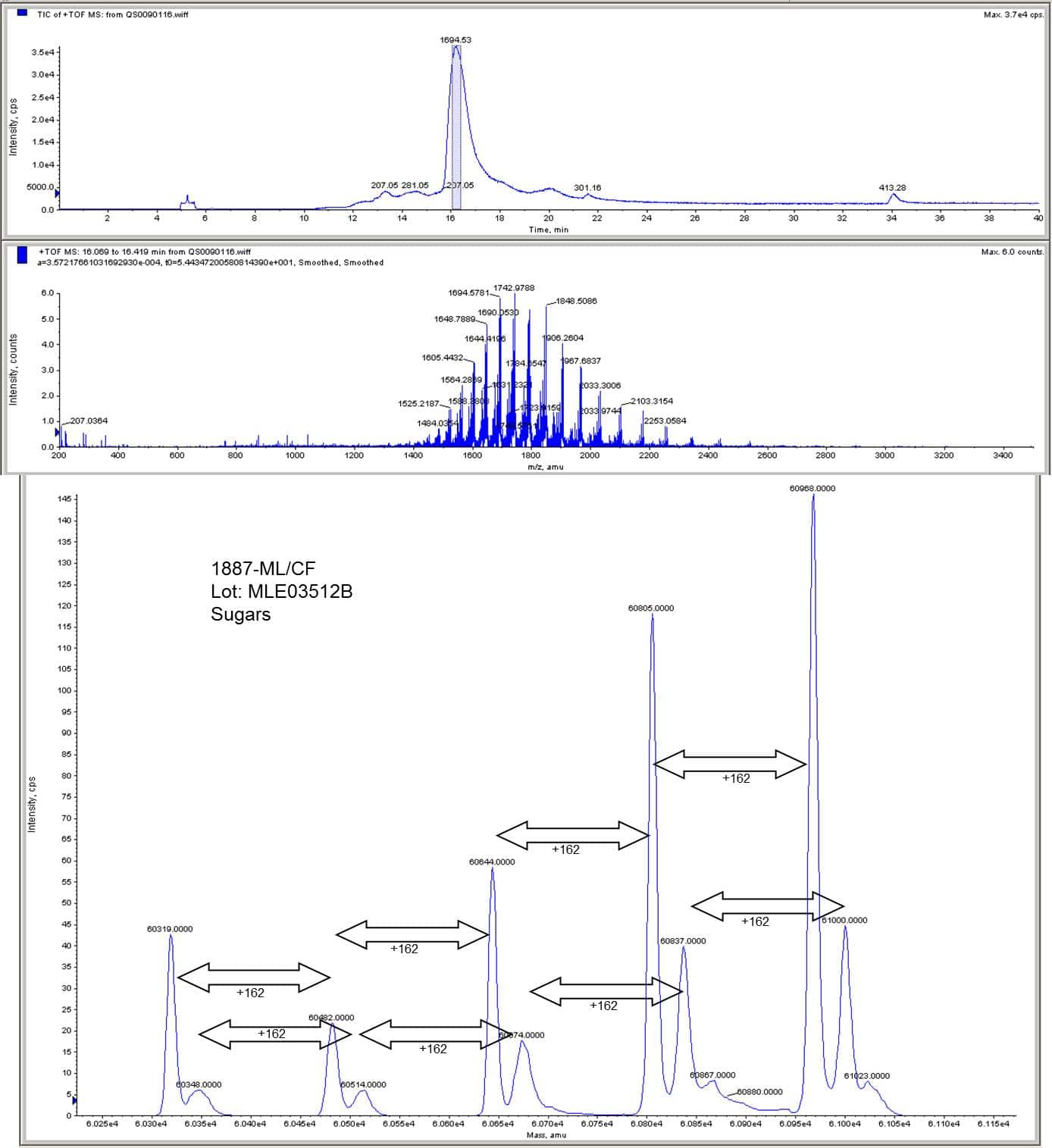
1887-ML/CF (carrier free)
Discontinued Product
1887-ML
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Reconstitution Calculator
Background: IL-23
Interleukin 23 (IL-23) is a heterodimeric cytokine composed of two disulfide-linked subunits, a p19 subunit that is unique to IL-23, and a p40 subunit that is shared with IL-12 (1-5). The p19 subunit has homology to the p35 subunit of IL-12, as well as to other single chain cytokines such as IL-6 and IL-11. The p40 subunit is homologous to the extracellular domains of the hematopoietic cytokine receptors. Mouse p19 cDNA encodes a 196 amino acid residue (aa) precursor protein with a putative 19 aa signal peptide and 177 aa mature protein. Human and mouse p19 share 70% aa sequence identity. Although p19 is expressed by activated macrophages, dendritic cells, T cells, and endothelial cells, only activated macrophages and dendritic cells express p40 concurrently to produce IL-23. The functional IL-23 receptor complex consists of two receptor subunits, the IL-12 receptor beta 1 subunit (IL-12 R beta 1) and the IL-23-specific receptor subunit (IL-23 R). IL-23 has biological activities that are similar to, but distinct from IL-12. Both IL-12 and IL-23 induce proliferation and IFN-gamma production by human T cells. While IL-12 acts on both naïve and memory human Tnbsp;cells, the effects of IL-23 is restricted to memory T cells. In mouse, IL-23 but not IL-12, has also been shown to induce memory T cells to secret IL-17, a potent proinflammatory cytokine. IL-12 and IL-23 can induce IL-12 production from mouse splenic DC of both the CD8- and CD8+ subtypes, however only IL-23 can act directly on CD8+ DC to mediate immunogenic presentation of poorly immunogenic tumor/self peptide.
- Oppmann, B. et al. (2000) Immunity 13:715.
- Lankford, C.S. and D.M. Frucht (2003) J. Leukoc. Biol. 73:49.
- Parham, C. et al. (2002) J. Immunol. 168:5699.
- Belladonna, M.L. et al. (2002) J. Immunol. 168:5448.
- Aggarwal, S. et al. (2003) J. Biol. Chem. 278:1910.
Citations for Recombinant Mouse IL-23 Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
186
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Sorafenib inhibits multiple sclerosis by regulating T cell differentiation
Authors: Wang, H;Wang, S;Wang, J;Fang, Y;Li, J;Shen, Y;Guo, J;
Cellular signalling
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-27 elicits a cytotoxic CD8+ T cell program to enforce tumour control
Authors: Bréart, B;Williams, K;Krimm, S;Wong, T;Kayser, BD;Wang, L;Cheng, E;Cruz Tleugabulova, M;Bouziat, R;Lu, T;Yuen, K;Firmino, NS;Bravo, DD;Roels, J;Bhakta, A;Bevers, J;Lehoux, I;Gutierrez, A;Chestnut, Y;Klementowicz, JE;Arenzana, TL;Akhmetzyanova, I;Dixon, E;Chen, M;Tasneem, K;Yadav, R;Koeppen, H;Oh, SA;Delamarre, L;Huang, H;Lim, SA;Nakamura, G;Wang, J;Gao, C;Corpuz, R;Müller, S;West, NR;
Nature
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Skin hepcidin initiates psoriasiform skin inflammation via Fe-driven hyperproliferation and neutrophil recruitment
Authors: Abboud, E;Chrayteh, D;Boussetta, N;Dalle, H;Malerba, M;Wu, TD;Le Gall, M;Reelfs, O;Pourzand, C;Mellett, M;Assan, F;Bachelez, H;Poupon, J;Aractingi, S;Vaulont, S;Sohier, P;Oules, B;Karim, Z;Peyssonnaux, C;
Nature communications
Species: Transgenic Mouse
Sample Types: In Vivo
Applications: In vivo assay -
Dysregulation of Wnt/?-catenin signaling contributes to intestinal inflammation through regulation of group 3 innate lymphoid cells
Authors: Hao, J;Liu, C;Gu, Z;Yang, X;Lan, X;Guo, X;
Nature communications
Species: Mouse
Sample Types: Whole Cells
-
LncRNA Neat1 targets NonO and miR-128-3p to promote antigen-specific Th17 cell responses and autoimmune inflammation
Authors: Chen, S;Wang, J;Zhang, K;Ma, B;Li, X;Wei, R;Nian, H;
Cell death & disease
Species: Mouse
Sample Types: Transfected Whole Cells, Whole Cells
Applications: Bioassay -
CD40L modulates CD4+ T cell activation through receptor for activated C kinase 1
Authors: van Os, BW;Vos, WG;Bosmans, LA;van Tiel, CM;Toom, MD;Beckers, L;Admiraal, M;Hoeksema, MA;de Winther, MP;Lutgens, E;
European journal of immunology
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Transcription factor EGR2 controls homing and pathogenicity of TH17 cells in the central nervous system
Authors: Gao, Y;Wang, Y;Chauss, D;Villarino, AV;Link, VM;Nagashima, H;Spinner, CA;Koparde, VN;Bouladoux, N;Abers, MS;Break, TJ;Chopp, LB;Park, JH;Zhu, J;Wiest, DL;Leonard, WJ;Lionakis, MS;O'Shea, JJ;Afzali, B;Belkaid, Y;Lazarevic, V;
Nature immunology
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Nsun2 coupling with RoRgammat shapes the fate of Th17 cells and promotes colitis
Authors: WL Yang, W Qiu, T Zhang, K Xu, ZJ Gu, Y Zhou, HJ Xu, ZZ Yang, B Shen, YL Zhao, Q Zhou, Y Yang, W Li, PY Yang, YG Yang
Nature Communications, 2023-02-16;14(1):863.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Cancer cells produce liver metastasis via gap formation in sinusoidal endothelial cells through proinflammatory paracrine mechanisms
Authors: T Huu Hoang, M Sato-Matsu, H Yuasa, T Matsubara, LTT Thuy, H Ikenaga, DM Phuong, NV Hanh, VN Hieu, DV Hoang, H Hai, Y Okina, M Enomoto, A Tamori, A Daikoku, H Urushima, K Ikeda, NQ Dat, Y Yasui, H Shinkawa, S Kubo, R Yamagishi, N Ohtani, K Yoshizato, J Gracia-San, N Kawada
Science Advances, 2022-09-28;8(39):eabo5525.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-3 regulates the differentiation of pathogenic Th17 cells
Authors: L Rani, A Kumar, J Karhade, G Pandey, A Guha, GC Mishra, MR Wani
European Journal of Immunology, 2022-09-26;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-23 signaling prevents ferroptosis-driven renal immunopathology during candidiasis
Authors: N Millet, NV Solis, D Aguilar, MS Lionakis, RT Wheeler, N Jendzjowsk, M Swidergall
Nature Communications, 2022-09-22;13(1):5545.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Antioxidative enzyme NAD(P)H quinone oxidoreductase 1 (NQO1) modulates the differentiation of Th17 cells by regulating ROS levels
Authors: K Nishida-Ta, A Kimura, T Tsubata, S Takahashi, H Suzuki
PLoS ONE, 2022-07-29;17(7):e0272090.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
pH sensing controls tissue inflammation by modulating cellular metabolism and endo-lysosomal function of immune cells
Authors: X Chen, A Jaiswal, Z Costliow, P Herbst, EA Creasey, N Oshiro-Rap, MJ Daly, KL Carey, DB Graham, RJ Xavier
Nature Immunology, 2022-06-06;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Acetylation licenses Th1 cell polarization to constrain Listeria monocytogenes infection
Authors: YS Zhang, DE Xin, Z Wang, W Peng, Y Zeng, J Liang, M Xu, N Chen, J Zhang, J Yue, M Cao, C Zhang, Y Wang, Z Chang, XM Lu, L Chang, YE Chinn
Cell Death and Differentiation, 2022-05-25;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
STING is an intrinsic checkpoint inhibitor that restrains the TH17 cell pathogenic program
Authors: LEA Damasceno, GCM Cebinelli, MF Fernandes, DC Nascimento, GA Públio, MAR Vinolo, SC Oliveira, T Sparwasser, TM Cunha, FQ Cunha, JC Alves-Filh
Cell Reports, 2022-05-24;39(8):110838.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TH17 cells promote CNS inflammation by sensing danger signals via Mincle
Authors: Q Zhang, W Liu, H Wang, H Zhou, K Bulek, X Chen, CJ Zhang, J Zhao, R Zhang, C Liu, Z Kang, RA Bermel, G Dubyak, DW Abbott, TS Xiao, LE Nagy, X Li
Nature Communications, 2022-05-03;13(1):2406.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Intestinal fibroblastic reticular cell niches control innate lymphoid cell homeostasis and function
Authors: HW Cheng, U Mörbe, M Lütge, C Engetschwi, L Onder, M Novkovic, C Gil-Cruz, C Perez-Shib, T Hehlgans, E Scandella, B Ludewig
Nature Communications, 2022-04-19;13(1):2027.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
CD4+ T-cell-derived IL-10 promotes CNS inflammation in mice by sustaining effector T�cell survival
Authors: N Yogev, T Bedke, Y Kobayashi, L Brockmann, D Lukas, T Regen, AL Croxford, A Nikolav, N Hövelmeyer, E von Stebut, M Prinz, C Ubeda, KJ Maloy, N Gagliani, RA Flavell, A Waisman, S Huber
Cell Reports, 2022-03-29;38(13):110565.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Promoting mechanism of serum amyloid a family expression in mouse intestinal epithelial cells
Authors: M Wakai, R Hayashi, Y Ueno, K Onishi, T Takasago, T Uchida, H Takigawa, R Yuge, Y Urabe, S Oka, Y Kitadai, S Tanaka
PLoS ONE, 2022-03-18;17(3):e0264836.
Species: Mouse
Sample Types: Organoid
Applications: Organoid Culture -
RORgammat phosphorylation protects against T�cell-mediated inflammation
Authors: S Ma, SA Patel, Y Abe, N Chen, PR Patel, BS Cho, N Abbasi, S Zeng, B Schnabl, JT Chang, WJM Huang
Cell Reports, 2022-03-15;38(11):110520.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Epigenetic drug ameliorated type 1 diabetes via decreased generation of Th1 and Th17 subsets and restoration of self-tolerance in CD4+ T cells
Authors: V Patel, A Jayaraman, S Jayaraman
International immunopharmacology, 2021-12-23;103(0):108490.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Allosteric inhibition of SHP2 uncovers aberrant TLR7 trafficking in aggravating psoriasis
Authors: Y Zhu, Z Wu, W Yan, F Shao, B Ke, X Jiang, J Gao, W Guo, Y Lai, H Ma, D Chen, Q Xu, Y Sun
Embo Molecular Medicine, 2021-12-22;0(0):e14455.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
G-protein-coupled receptor P2Y10 facilitates chemokine-induced CD4 T cell migration through autocrine/paracrine mediators
Authors: M Gurusamy, D Tischner, J Shao, S Klatt, S Zukunft, R Bonnavion, S Günther, K Siebenbrod, RI Kestner, T Kuhlmann, I Fleming, S Offermanns, N Wettschure
Nature Communications, 2021-11-23;12(1):6798.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
RORalpha is critical for mTORC1 activity in T�cell-mediated colitis
Authors: X Chi, W Jin, X Bai, X Zhao, J Shao, J Li, Q Sun, B Su, X Wang, XO Yang, C Dong
Cell Reports, 2021-09-14;36(11):109682.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interferon-beta Intensifies Interleukin-23-Driven Pathogenicity of T Helper Cells in Neuroinflammatory Disease
Authors: A Agasing, JL Quinn, G Kumar, RC Axtell
Cells, 2021-08-20;10(8):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
m6A demethylase ALKBH5 controls CD4+ T cell pathogenicity and promotes autoimmunity
Authors: J Zhou, X Zhang, J Hu, R Qu, Z Yu, H Xu, H Chen, L Yan, C Ding, Q Zou, Y Ye, Z Wang, RA Flavell, HB Li
Science Advances, 2021-06-16;7(25):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Ontogenic timing, T�cell receptor signal strength, and Notch signaling direct &gamma&delta T�cell functional differentiation in�vivo
Authors: ELY Chen, CR Lee, PK Thompson, DL Wiest, MK Anderson, JC Zúñiga-Pfl
Cell Reports, 2021-06-08;35(10):109227.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
NLRP1 acts as a negative regulator of Th17 cell programming in mice and humans with autoimmune diabetes
Authors: FRC Costa, JA Leite, DM Rassi, JF da Silva, J Elias-Oliv, JB Guimarães, MC Foss-Freit, NOS Câmara, A Pontillo, RC Tostes, JS Silva, D Carlos
Cell Reports, 2021-05-25;35(8):109176.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Differential controls of MAIT cell effector polarization by mTORC1/mTORC2 via integrating cytokine and costimulatory signals
Authors: H Tao, Y Pan, S Chu, L Li, J Xie, P Wang, S Zhang, S Reddy, JW Sleasman, XP Zhong
Nature Communications, 2021-04-01;12(1):2029.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
Pertussis Toxin Inhibits Encephalitogenic T-Cell Infiltration and Promotes a B-Cell-Driven Disease during Th17-EAE
Authors: Z Maria, E Turner, A Agasing, G Kumar, RC Axtell
International Journal of Molecular Sciences, 2021-03-13;22(6):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Male sex chromosomal complement exacerbates the pathogenicity of Th17 cells in a chronic model of central nervous system autoimmunity
Authors: PMIA Doss, M Umair, J Baillargeo, R Fazazi, N Fudge, I Akbar, AP Yeola, JB Williams, M Leclercq, C Joly-Beaup, P Beauchemin, GF Ruda, M Alpaugh, AC Anderson, PE Brennan, A Droit, H Lassmann, CS Moore, M Rangachari
Cell Reports, 2021-03-09;34(10):108833.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TARM1 contributes to development of arthritis by activating dendritic cells through recognition of collagens
Authors: R Yabe, SH Chung, MA Murayama, S Kubo, K Shimizu, Y Akahori, T Maruhashi, A Seno, T Kaifu, S Saijo, Y Iwakura
Nature Communications, 2021-01-04;12(1):94.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
An IL-27-Driven Transcriptional Network Identifies Regulators of IL-10 Expression across T Helper Cell Subsets
Authors: H Zhang, A Madi, N Yosef, N Chihara, A Awasthi, C Pot, C Lambden, A Srivastava, PR Burkett, J Nyman, E Christian, Y Etminan, A Lee, H Stroh, J Xia, K Karwacz, PI Thakore, N Acharya, A Schnell, C Wang, L Apetoh, O Rozenblatt, AC Anderson, A Regev, VK Kuchroo
Cell Rep, 2020-11-24;33(8):108433.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
The frequency of follicular T helper cells differs in acute and chronic neuroinflammation
Authors: A Baniahmad, K Birkner, J Görg, J Loos, F Zipp, B Wasser, S Bittner
Sci Rep, 2020-11-24;10(1):20485.
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Synthetic lethality between MyD88 loss and mutations in Wnt/&beta-catenin pathway in intestinal tumor epithelial cells
Authors: R Kajino-Sak, T Fujishita, MM Taketo, M Aoki
Oncogene, 2020-11-12;0(0):.
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
EBV-induced gene 3 augments IL-23R&alpha protein expression through a chaperone calnexin
Authors: I Mizoguchi, M Ohashi, H Hasegawa, Y Chiba, N Orii, S Inoue, C Kawana, M Xu, K Sudo, K Fujita, M Kuroda, SI Hashimoto, K Matsushima, T Yoshimoto
J. Clin. Invest., 2020-11-02;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
ADAM12 is a costimulatory molecule that determines Th1 cell fate and mediates tissue inflammation
Authors: Y Liu, R Bockermann, M Hadi, I Safari, B Carrion, M Kveiborg, S Issazadeh-
Cell. Mol. Immunol., 2020-06-22;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Transcriptomics and proteomics reveal a cooperation between interferon and T-helper 17 cells in neuromyelitis optica
Authors: AM Agasing, Q Wu, B Khatri, N Borisow, K Ruprecht, AU Brandt, S Gawde, G Kumar, JL Quinn, RM Ko, Y Mao-Draaye, CJ Lessard, F Paul, RC Axtell
Nat Commun, 2020-06-05;11(1):2856.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-23 in arthritic and inflammatory pain development in mice
Authors: KM Lee, Z Zhang, A Achuthan, AJ Fleetwood, JE Smith, JA Hamilton, AD Cook
Arthritis Res. Ther., 2020-05-29;22(1):123.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Targeting cellular fatty acid synthesis limits T helper and innate lymphoid cell function during intestinal inflammation and infection
Authors: P Mamareli, F Kruse, CW Lu, M Guderian, S Floess, K Rox, DSJ Allan, JR Carlyle, M Brönstrup, R Müller, L Berod, T Sparwasser, M Lochner
Mucosal Immunol, 2020-04-30;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Neurokinin-1 Receptor Signaling Is Required for Efficient Ca2+ Flux in T-Cell-Receptor-Activated T Cells
Authors: AE Morelli, TL Sumpter, DM Rojas-Cana, M Bandyopadh, Z Chen, O Tkacheva, WJ Shufesky, CT Wallace, SC Watkins, A Berger, CJ Paige, LD Falo, AT Larregina
Cell Rep, 2020-03-10;30(10):3448-3465.e8.
Species: Mouse
Sample Types: Dendritic Cells
Applications: Bioassay -
Prognostic value of serum soluble interleukin-23 receptor and related T-helper 17 cell cytokines in non-small cell lung carcinoma
Authors: D Liu, S Xing, W Wang, X Huang, H Lin, Y Chen, K Lan, L Chen, F Luo, S Qin, R Liang, C Bai, J Xu, W Liu
Cancer Sci., 2020-03-04;111(4):1093-1102.
Applications: ELISA Developmet -
Anxiolytic Drug FGIN-1-27 Ameliorates Autoimmunity by Metabolic Reprogramming of Pathogenic Th17 Cells
Authors: A Singh, M Dashnyam, B Chim, TM Escobar, AE Dulcey, X Hu, KM Wilson, PP Koganti, CA Spinner, X Xu, A Jadhav, N Southall, J Marugan, V Selvaraj, V Lazarevic, SA Muljo, M Ferrer
Sci Rep, 2020-02-28;10(1):3766.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
TNFR2 limits proinflammatory astrocyte functions during EAE induced by pathogenic DR2b-restricted T cells
Authors: I Raphael, F Gomez-Rive, RA Raphael, RR Robinson, S Nalawade, TG Forsthuber
JCI Insight, 2019-12-19;4(24):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
CD4+ CCR6+ T�cells, but not gammadelta T�cells, are important for the IL-23R-dependent progression of antigen-induced inflammatory arthritis in mice
Authors: W Razawy, PS Asmawidjaj, AM Mus, N Salioska, N Davelaar, N Kops, M Oukka, CH Alves, E Lubberts
Eur. J. Immunol., 2019-11-28;50(2):245-255.
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
NFAT-Specific Inhibition by dNP2-VIVITAmeliorates Autoimmune Encephalomyelitisby Regulation of Th1 and Th17
Authors: HG Lee, LK Kim, JM Choi
Mol Ther Methods Clin Dev, 2019-10-23;16(0):32-41.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
NLRP6 Deficiency in CD4 T Cells Decreases T Cell Survival Associated with Increased Cell Death
Authors: K Radulovic, CK Ayata, R Mak'Anyeng, K Lechner, P Wuggenig, B Kaya, P Hruz, M Gomez de A, P Broz, B Weigmann, JH Niess
J. Immunol., 2019-05-31;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
cIAP1/2 inhibition synergizes with TNF inhibition in autoimmunity by down-regulating IL-17A and inducing Tregs
Authors: JZ Kawalkowsk, J Ogbechi, PJ Venables, RO Williams
Sci Adv, 2019-05-01;5(5):eaaw5422.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Activation of aryl hydrocarbon receptor signaling by a novel agonist ameliorates autoimmune encephalomyelitis
Authors: A Abdullah, M Maged, I Hairul-Isl, A Osama I, H Maha, A Manal, H Hamza
PLoS ONE, 2019-04-26;14(4):e0215981.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Retinal microglia initiate neuroinflammation in ocular autoimmunity
Authors: Y Okunuki, R Mukai, T Nakao, SJ Tabor, O Butovsky, R Dana, BR Ksander, KM Connor
Proc. Natl. Acad. Sci. U.S.A., 2019-04-25;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-1 and TNF? Contribute to the Inflammatory Niche to Enhance Alveolar Regeneration
Authors: H Katsura, Y Kobayashi, PR Tata, BLM Hogan
Stem Cell Reports, 2019-03-28;0(0):.
Species: Mouse
Sample Types: Organoids
Applications: Bioassay -
NCR- group 3 innate lymphoid cells orchestrate IL-23/IL-17 axis to promote hepatocellular carcinoma development
Authors: Y Liu, Y Song, D Lin, L Lei, Y Mei, Z Jin, H Gong, Y Zhu, B Hu, Y Zhang, L Zhao, HY Teo, J Qiu, W Jiang, C Dong, D Wu, Y Huang, H Liu
EBioMedicine, 2019-03-01;0(0):.
Species: Mouse
Sample Types: Whole Cells
-
Pathogenic function of bystander-activated memory-like CD4+ T cells in autoimmune encephalomyelitis
Authors: HG Lee, JU Lee, DH Kim, S Lim, I Kang, JM Choi
Nat Commun, 2019-02-12;10(1):709.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
DGAT1 inhibits retinol-dependent regulatory T cell formation and mediates autoimmune encephalomyelitis
Authors: KL Graham, BJ Werner, KM Moyer, AK Patton, CR Krois, HS Yoo, M Tverskoy, M LaJevic, JL Napoli, RA Sobel, BA Zabel, EC Butcher
Proc. Natl. Acad. Sci. U.S.A., 2019-02-04;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Satb1 regulates the effector program of encephalitogenic tissue Th17 cells in chronic inflammation
Authors: K Yasuda, Y Kitagawa, R Kawakami, Y Isaka, H Watanabe, G Kondoh, T Kohwi-Shig, S Sakaguchi, K Hirota
Nat Commun, 2019-02-01;10(1):549.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
REV-ERB? Regulates TH17 Cell Development and Autoimmunity
Authors: M Amir, S Chaudhari, R Wang, S Campbell, SA Mosure, LB Chopp, Q Lu, J Shang, OB Pelletier, Y He, C Doebelin, MD Cameron, DJ Kojetin, TM Kamenecka, LA Solt
Cell Rep, 2018-12-26;25(13):3733-3749.e8.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TGF?1 signaling sustains aryl hydrocarbon receptor (AHR) expression and restrains the pathogenic potential of TH17 cells by an AHR-independent mechanism
Authors: KA de Lima, PB Donate, J Talbot, M Davoli-Fer, RS Peres, TM Cunha, JC Alves-Filh, FQ Cunha
Cell Death Dis, 2018-11-13;9(11):1130.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TLR-stimulated IRAKM activates caspase-8 inflammasome in microglia and promotes neuroinflammation
Authors: CJ Zhang, M Jiang, H Zhou, W Liu, C Wang, Z Kang, B Han, Q Zhang, X Chen, J Xiao, A Fisher, WJ Kaiser, MA Murayama, Y Iwakura, J Gao, J Carman, A Dongre, G Dubyak, DW Abbott, FD Shi, RM Ransohoff, X Li
J. Clin. Invest., 2018-10-29;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The Citrobacter rodentium type III secretion system effector EspO affects mucosal damage repair and antimicrobial responses
Authors: CN Berger, VF Crepin, TI Roumelioti, JC Wright, N Serafini, M Pevsner-Fi, L Yu, E Elinav, JP Di Santo, JS Choudhary, G Frankel
PLoS Pathog., 2018-10-26;14(10):e1007406.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Disruption of FOXP3-EZH2 Interaction Represents a Pathobiological Mechanism in Intestinal Inflammation
Authors: AO Bamidele, PA Svingen, MR Sagstetter, OF Sarmento, M Gonzalez, MB Braga Neto, S Kugathasan, G Lomberk, RA Urrutia, WA Faubion
Cell Mol Gastroenterol Hepatol, 2018-09-14;7(1):55-71.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
GLK-IKK? signaling induces dimerization and translocation of the AhR-ROR?t complex in IL-17A induction and autoimmune disease
Authors: HC Chuang, CY Tsai, CH Hsueh, TH Tan
Sci Adv, 2018-09-12;4(9):eaat5401.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Blockade of MCAM/CD146 impedes CNS infiltration of T cells over the choroid plexus
Authors: J Breuer, E Korpos, MJ Hannocks, T Schneider-, J Song, L Zondler, S Herich, K Flanagan, T Korn, A Zarbock, T Kuhlmann, L Sorokin, H Wiendl, N Schwab
J Neuroinflammation, 2018-08-22;15(1):236.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
??TCR regulates production of interleukin-27 by neutrophils and attenuates inflammatory arthritis
Authors: L Bouchareyc, EM Grössinger, M Kang, IE Adamopoulo
Sci Rep, 2018-05-15;8(1):7590.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A cytokine network involving IL-36?, IL-23, and IL-22 promotes antimicrobial defense and recovery from intestinal barrier damage
Authors: VL Ngo, H Abo, E Maxim, A Harusato, D Geem, O Medina-Con, D Merlin, AT Gewirtz, A Nusrat, TL Denning
Proc. Natl. Acad. Sci. U.S.A., 2018-05-14;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation
Authors: R Wu, J Zeng, J Yuan, X Deng, Y Huang, L Chen, P Zhang, H Feng, Z Liu, Z Wang, X Gao, H Wu, H Wang, Y Su, M Zhao, Q Lu
J. Clin. Invest., 2018-05-14;0(0):.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
NCR+ ILC3 maintain larger STAT4 reservoir via T-BET to regulate type 1 features upon IL-23 stimulation in mice
Authors: Y Mikami, G Scarno, B Zitti, HY Shih, Y Kanno, A Santoni, JJ O'Shea, G Sciumè
Eur. J. Immunol., 2018-04-16;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Epigenetic activation during T helper 17 cell differentiation is mediated by Tripartite motif containing 28
Authors: Y Jiang, Y Liu, H Lu, SC Sun, W Jin, X Wang, C Dong
Nat Commun, 2018-04-12;9(1):1424.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Fas Promotes T Helper 17 Cell Differentiation and Inhibits T Helper 1 Cell Development by Binding and Sequestering Transcription Factor STAT1
Authors: G Meyer Zu H, D Przybylski, MA Schramm, C Wang, A Schnell, Y Lee, R Sobel, A Regev, VK Kuchroo
Immunity, 2018-03-20;48(3):556-569.e7.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-25 enhances Th17 cell-mediated contact dermatitis by promoting IL-1? production by dermal dendritic cells
Authors: H Suto, A Nambu, H Morita, S Yamaguchi, T Numata, T Yoshizaki, E Shimura, K Arae, Y Asada, K Motomura, M Kaneko, T Abe, A Matsuda, Y Iwakura, K Okumura, H Saito, K Matsumoto, K Sudo, S Nakae
J. Allergy Clin. Immunol., 2018-03-06;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-23-induced macrophage polarization and its pathological roles in mice with imiquimod-induced psoriasis
Authors: Y Hou, L Zhu, H Tian, HX Sun, R Wang, L Zhang, Y Zhao
Protein Cell, 2018-03-05;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Characterization and allergic role of IL-33-induced neutrophil polarization
Authors: B Sun, L Zhu, Y Tao, HX Sun, Y Li, P Wang, Y Hou, Y Zhao, X Zhang, L Zhang, N Na, Y Zhao
Cell. Mol. Immunol., 2018-03-05;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
STAT-3-independent production of IL-17 by mouse innate-like ?? T cells controls ocular infection
Authors: AJ St Leger, AM Hansen, H Karauzum, R Horai, CR Yu, A Laurence, KD Mayer-Barb, P Silver, R Villasmil, C Egwuagu, SK Datta, RR Caspi
J. Exp. Med., 2018-02-28;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Oxysterol Sensing through the Receptor GPR183 Promotes the Lymphoid-Tissue-Inducing Function of Innate Lymphoid Cells and Colonic Inflammation
Authors: J Emgård, H Kammoun, B García-Cas, J Chesné, SM Parigi, JM Jacob, HW Cheng, E Evren, S Das, P Czarnewski, N Sleiers, F Melo-Gonza, E Kvedaraite, M Svensson, E Scandella, MR Hepworth, S Huber, B Ludewig, L Peduto, EJ Villablanc, H Veiga-Fern, JP Pereira, RA Flavell, T Willinger
Immunity, 2018-01-16;48(1):120-132.e8.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
GM-CSF Promotes Chronic Disability in Experimental Autoimmune Encephalomyelitis by Altering the Composition of Central Nervous System-Infiltrating Cells, but Is Dispensable for Disease Induction
Authors: PC Duncker, JS Stoolman, AK Huber, BM Segal
J. Immunol., 2017-12-29;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Protein Tyrosine Phosphatase PTPN22 regulates IL-1? dependent Th17 responses by modulating dectin-1 signaling in mice
Authors: HA Purvis, F Clarke, CK Jordan, CS Blanco, GH Cornish, X Dai, DJ Rawlings, R Zamoyska, AP Cope
Eur. J. Immunol., 2017-10-20;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-17A-promoted MSC2 polarization related with new bone formation of ankylosing spondylitis
Authors: T He, Y Huang, C Zhang, D Liu, C Cheng, W Xu, X Zhang
Oncotarget, 2017-09-11;8(57):96993-97008.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-1? and IL-23 Promote Extrathymic Commitment of CD27(+)CD122(-) ?? T Cells to ??T17 Cells
Authors: A Muschaweck, F Petermann, T Korn
J. Immunol., 2017-08-30;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Brain micro-inflammation at specific vessels dysregulates organ-homeostasis via the activation of a new neural circuit
Authors: Y Arima, T Ohki, N Nishikawa, K Higuchi, M Ota, Y Tanaka, J Nio-Kobaya, M Elfeky, R Sakai, Y Mori, T Kawamoto, A Stofkova, Y Sakashita, Y Morimoto, M Kuwatani, T Iwanaga, Y Yoshioka, N Sakamoto, A Yoshimura, M Takiguchi, S Sakoda, M Prinz, D Kamimura, M Murakami
Elife, 2017-08-15;6(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The Development of Steady-State Activation Hubs between Adult LTi ILC3s and Primed Macrophages in Small Intestine
Authors: AK Savage, HE Liang, RM Locksley
J. Immunol., 2017-07-26;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
The STAT4/MLL1 Epigenetic Axis Regulates the Antimicrobial Functions of Murine Macrophages
Authors: WF Carson, KA Cavassani, EM Soares, S Hirai, NA Kittan, MA Schaller, MM Scola, A Joshi, A Matsukawa, DM Aronoff, CN Johnson, Y Dou, KA Gallagher, SL Kunkel
J. Immunol., 2017-07-21;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The excretory-secretory products of Echinococcus granulosus protoscoleces directly regulate the differentiation of B10, B17 and Th17 cells
Authors: W Pan, WT Hao, YJ Shen, XY Li, YJ Wang, FF Sun, JH Yin, J Zhang, RX Tang, JP Cao, KY Zheng
Parasit Vectors, 2017-07-21;10(1):348.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Hypomorphic A20 expression confers susceptibility to psoriasis
Authors: A Aki, M Nagasaki, BA Malynn, A Ma, T Kagari
PLoS ONE, 2017-06-28;12(6):e0180481.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
IL-23 Limits the Production of IL-2 and Promotes Autoimmunity in Lupus
Authors: H Dai, F He, GC Tsokos, VC Kyttaris
J. Immunol., 2017-06-23;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Microbiota-derived butyrate suppresses group 3 innate lymphoid cells in terminal ileal Peyer's patches
Authors: SH Kim, BH Cho, H Kiyono, YS Jang
Sci Rep, 2017-06-21;7(1):3980.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A Distinct Inhibitory Function for miR-18a in Th17 Cell Differentiation
Authors: MM Montoya, J Maul, PB Singh, HH Pua, F Dahlström, N Wu, X Huang, KM Ansel, D Baumjohann
J. Immunol., 2017-06-12;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The frequency of Th17�cells in the small intestine exhibits a day-night variation dependent on circadian clock activity
Authors: HP Thu Le, Y Nakamura, K Oh-Oka, K Ishimaru, S Nakajima, A Nakao
Biochem. Biophys. Res. Commun., 2017-06-12;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Viral RNA-Unprimed Rig-I Restrains Stat3 Activation in the Modulation of Regulatory T Cell/Th17 Cell Balance
Authors: H Yang, HZ Guo, XY Li, J Lin, W Zhang, JM Zhao, HX Zhang, SJ Chen, Z Chen, J Zhu
J. Immunol., 2017-05-26;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Selective Induction of Homeostatic Th17 Cells in the Murine Intestine by Cholera Toxin Interacting with the Microbiota
Authors: Q Zhao, SN Harbour, R Kolde, IJ Latorre, HM Tun, TR Schoeb, H Turner, JJ Moon, E Khafipour, RJ Xavier, CT Weaver, CO Elson
J. Immunol., 2017-05-24;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
RIG-I antiviral signaling drives interleukin-23 production and psoriasis-like skin disease
Authors: H Zhu, F Lou, Q Yin, Y Gao, Y Sun, J Bai, Z Xu, Z Liu, W Cai, F Ke, L Zhang, H Zhou, H Wang, G Wang, X Chen, H Zhang, Z Wang, F Ginhoux, C Lu, B Su, H Wang
EMBO Mol Med, 2017-05-01;9(5):589-604.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Eomes expression reports the progressive differentiation of IFN-?-producing Th1-like ?? T cells
Authors: CN Lino, J Barros-Mar, L Oberdörfer, T Walzer, I Prinz
Eur. J. Immunol., 2017-04-24;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Suppression of Th17 cell differentiation by misshapen/NIK-related kinase MINK1
Authors: G Fu, Q Xu, Y Qiu, X Jin, T Xu, S Dong, J Wang, Y Ke, H Hu, X Cao, D Wang, H Cantor, X Gao, L Lu
J. Exp. Med., 2017-04-11;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
GM-CSF is not essential for experimental autoimmune encephalomyelitis but promotes brain-targeted disease
Authors: ER Pierson, JM Goverman
JCI Insight, 2017-04-06;2(7):e92362.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Suppressive IL-17A(+)Foxp3(+) and ex-Th17 IL-17A(neg)Foxp3(+) Treg cells are a source of tumour-associated Treg cells
Authors: S Downs-Cann, S Berkey, GM Delgoffe, RP Edwards, T Curiel, K Odunsi, DL Bartlett, N Obermajer
Nat Commun, 2017-03-14;8(0):14649.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Inhibiting Oxidative Phosphorylation In Vivo Restrains Th17 Effector Responses and Ameliorates Murine Colitis
Authors: L Franchi, I Monteleone, LY Hao, MA Spahr, W Zhao, X Liu, K Demock, A Kulkarni, CA Lesch, B Sanchez, L Carter, I Marafini, X Hu, O Mashadova, M Yuan, JM Asara, H Singh, CA Lyssiotis, G Monteleone, AW Opipari, GD Glick
J. Immunol, 2017-02-27;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
K48-linked KLF4 ubiquitination by E3 ligase Mule controls T-cell proliferation and cell cycle progression
Authors: Z Hao, Y Sheng, GS Duncan, WY Li, C Dominguez, J Sylvester, YW Su, GH Lin, BE Snow, D Brenner, A You-Ten, J Haight, S Inoue, A Wakeham, A Elford, S Hamilton, Y Liang, JC Z£¤iga-Pfl, HH He, PS Ohashi, TW Mak
Nat Commun, 2017-01-13;8(0):14003.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Dual role of ALCAM in neuroinflammation and blood-brain barrier homeostasis
Authors: MA L‚cuyer, O Saint-Laur, L BourbonniŠ, S Larouche, C Larochelle, L Michel, M Charabati, M Abadier, S Zandee, N Haghayegh, E Gowing, C Pittet, R Lyck, B Engelhardt, A Prat
Proc. Natl. Acad. Sci. U.S.A, 2017-01-09;114(4):E524-E533.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Ndfip1 restricts Th17 cell potency by limiting lineage stability and proinflammatory cytokine production
Authors: AA Kesewa Lay, S L Sprout, D Phillips, PM Oliver
Sci Rep, 2017-01-04;7(0):39649.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Pharmacologic modulation of ROR?t translates to efficacy in preclinical and translational models of psoriasis and inflammatory arthritis
Sci Rep, 2016-12-01;6(0):37977.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
Keratinocytes contribute intrinsically to psoriasis upon loss of Tnip1 function
Proc Natl Acad Sci USA, 2016-09-26;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Dose-Dependent Suppression of Cytokine production from T cells by a Novel Phosphoinositide 3-Kinase Delta Inhibitor
Sci Rep, 2016-07-27;6(0):30384.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
SLAM-associated protein favours the development of iNKT2 over iNKT17 cells
Authors: Marie-Laure Michel
Eur J Immunol, 2016-07-22;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Attenuation of PKR-like ER Kinase (PERK) Signaling Selectively Controls Endoplasmic Reticulum Stress-induced Inflammation Without Compromising Immunological Responses
Authors: LN Guthrie, K Abiraman, ES Plyler, NT Sprenkle, SA Gibson, BC McFarland, R Rajbhandar, AL Rowse, EN Benveniste, GP Meares
J. Biol. Chem., 2016-05-23;291(30):15830-40.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Blockade of Extracellular ATP Effect by Oxidized ATP Effectively Mitigated Induced Mouse Experimental Autoimmune Uveitis (EAU)
PLoS ONE, 2016-05-19;11(5):e0155953.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Phenotypic and Functional Plasticity of Murine Intestinal NKp46+ Group 3 Innate Lymphoid Cells
J Immunol, 2016-04-22;196(11):4731-8.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Ndfip-mediated degradation of Jak1 tunes cytokine signalling to limit expansion of CD4+ effector T cells
Authors: CE O'Leary, CR Riling, LA Spruce, H Ding, S Kumar, G Deng, Y Liu, SH Seeholzer, PM Oliver
Nat Commun, 2016-04-18;7(0):11226.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Myelin-specific Th17 cells induce severe relapsing optic neuritis with irreversible loss of retinal ganglion cells in C57BL/6 mice
Authors: CM Larabee, Y Hu, S Desai, C Georgescu, JD Wren, RC Axtell, SM Plafker
Mol Vis, 2016-04-11;22(0):332-41.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Toll/Interleukin-1 Receptor Domain Derived from TcpC (TIR-TcpC) Ameliorates Experimental Autoimmune Arthritis by Down-modulating Th17 Cell Response
Authors: Shweta Pasi
J Biol Chem, 2016-03-28;291(23):12358-69.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-1-induced Bhlhe40 identifies pathogenic T helper cells in a model of autoimmune neuroinflammation.
Authors: Chih-Chung Lin, Tara R Bradstree, Elizabeth A Schwarzko, Nicholas N Jarjour, Chun Chou, Angela S Archambau, Julia Sim, Bernd H Zinselmey, Javier A Carrero, Gregory F Wu, Reshma Taneja, Maxim N Artyomov, John H Russell, Brian T Edelson
The Journal of Experimental Medicine, 2016-02-01;0(0):1540-9538.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
RGS10 deficiency ameliorates the severity of disease in experimental autoimmune encephalomyelitis.
Authors: Lee J, Kannarkat G, Chung J, Joon Lee H, Graham K, Tansey M
J Neuroinflammation, 2016-02-01;13(1):24.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
CD43 Functions as an E-Selectin Ligand for Th17 Cells In Vitro and Is Required for Rolling on the Vascular Endothelium and Th17 Cell Recruitment during Inflammation In Vivo.
Authors: Velazquez F, Grodecki-Pena A, Knapp A, Salvador A, Nevers T, Croce K, Alcaide P
J Immunol, 2015-12-23;196(3):1305-16.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-36gamma is expressed by neutrophils and can activate microglia, but has no role in experimental autoimmune encephalomyelitis.
Authors: Bozoyan L, Dumas A, Patenaude A, Vallieres L
J Neuroinflammation, 2015-09-17;12(0):173.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Composition, Development, and Function of Uterine Innate Lymphoid Cells.
Authors: Doisne J, Balmas E, Boulenouar S, Gaynor L, Kieckbusch J, Gardner L, Hawkes D, Barbara C, Sharkey A, Brady H, Brosens J, Moffett A, Colucci F
J Immunol, 2015-09-14;195(8):3937-45.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
An A2B Adenosine Receptor Agonist Promotes Th17 Autoimmune Responses in Experimental Autoimmune Uveitis (EAU) via Dendritic Cell Activation.
Authors: Chen M, Liang D, Zuo A, Shao H, Kaplan H, Sun D
PLoS ONE, 2015-07-06;10(7):e0132348.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Adaptive Immune-like gamma/delta T Lymphocytes Share Many Common Features with Their alpha/beta T Cell Counterparts.
Authors: Lombes A, Durand A, Charvet C, Riviere M, Bonilla N, Auffray C, Lucas B, Martin B
J Immunol, 2015-06-29;195(4):1449-58.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Cutting edge: c-Kit signaling differentially regulates type 2 innate lymphoid cell accumulation and susceptibility to central nervous system demyelination in male and female SJL mice.
Authors: Russi A, Walker-Caulfield M, Ebel M, Brown M
J Immunol, 2015-05-13;194(12):5609-13.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells.
Authors: Kim Y, Lim H, Jung H, Wetsel R, Chung Y
PLoS ONE, 2015-03-13;10(3):e0120294.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Tolerance of activated pathogenic CD4+ T cells by transcriptional targeting of dendritic cells.
Authors: de Andrade Pereira B, Ackermann M, Chaudhary S, Vogel R, Vogt B, Dresch C, Fraefel C
Gene Ther, 2015-03-05;22(5):382-90.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TGF-beta3-expressing CD4+CD25(-)LAG3+ regulatory T cells control humoral immune responses.
Authors: Okamura T, Sumitomo S, Morita K, Iwasaki Y, Inoue M, Nakachi S, Komai T, Shoda H, Miyazaki J, Fujio K, Yamamoto K
Nat Commun, 2015-02-19;6(0):6329.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
CXCL13 antibody for the treatment of autoimmune disorders.
Authors: Klimatcheva E, Pandina T, Reilly C, Torno S, Bussler H, Scrivens M, Jonason A, Mallow C, Doherty M, Paris M, Smith E, Zauderer M
BMC Immunol, 2015-02-12;16(0):6.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Testosterone suppresses hepatic inflammation by the downregulation of IL-17, CXCL-9, and CXCL-10 in a mouse model of experimental acute cholangitis.
Authors: Schwinge D, Carambia A, Quaas A, Krech T, Wegscheid C, Tiegs G, Prinz I, Lohse A, Herkel J, Schramm C
J Immunol, 2015-02-11;194(6):2522-30.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection.
Authors: Munoz M, Eidenschenk C, Ota N, Wong K, Lohmann U, Kuhl A, Wang X, Manzanillo P, Li Y, Rutz S, Zheng Y, Diehl L, Kayagaki N, van Lookeren-Campagne M, Liesenfeld O, Heimesaat M, Ouyang W
Immunity, 2015-02-10;42(2):321-31.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the TH17 cell-iTreg cell balance.
Authors: Basu R, Whitley S, Bhaumik S, Zindl C, Schoeb T, Benveniste E, Pear W, Hatton R, Weaver C
Nat Immunol, 2015-02-02;16(3):286-95.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge.
Authors: Monin L, Griffiths K, Slight S, Lin Y, Rangel-Moreno J, Khader S
Mucosal Immunol, 2015-01-28;8(5):1099-109.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood-brain barrier.
Authors: Abadier M, Haghayegh Jahromi N, Cardoso Alves L, Boscacci R, Vestweber D, Barnum S, Deutsch U, Engelhardt B, Lyck R
Eur J Immunol, 2015-01-23;45(4):1043-58.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
miR-148a is upregulated by Twist1 and T-bet and promotes Th1-cell survival by regulating the proapoptotic gene Bim.
Authors: Haftmann C, Stittrich A, Zimmermann J, Fang Z, Hradilkova K, Bardua M, Westendorf K, Heinz G, Riedel R, Siede J, Lehmann K, Weinberger E, Zimmel D, Lauer U, Haupl T, Sieper J, Backhaus M, Neumann C, Hoffmann U, Porstner M, Chen W, Grun J, Baumgrass R, Matz M, Lohning M, Scheffold A, Wittmann J, Chang H, Rajewsky N, Jack H, Radbruch A, Mashreghi M
Eur J Immunol, 2015-01-22;45(4):1192-205.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Temporal expression of growth factors triggered by epiregulin regulates inflammation development.
Authors: Harada M, Kamimura D, Arima Y, Kohsaka H, Nakatsuji Y, Nishida M, Atsumi T, Meng J, Bando H, Singh R, Sabharwal L, Jiang J, Kumai N, Miyasaka N, Sakoda S, Yamauchi-Takihara K, Ogura H, Hirano T, Murakami M
J Immunol, 2015-01-02;194(3):1039-46.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine.
Authors: Dunne A, Mielke L, Allen A, Sutton C, Higgs R, Cunningham C, Higgins S, Mills K
Mucosal Immunol, 2014-10-15;8(3):607-17.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Dysbiosis caused by vitamin D receptor deficiency confers colonization resistance to Citrobacter rodentium through modulation of innate lymphoid cells.
Authors: Chen J, Waddell A, Lin Y, Cantorna M
Mucosal Immunol, 2014-10-15;8(3):618-26.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Mesenchymal stem cells differentially modulate effector CD8+ T cell subsets and exacerbate experimental autoimmune encephalomyelitis.
Authors: Glenn J, Smith M, Calabresi P, Whartenby K
Stem Cells, 2014-10-01;32(10):2744-55.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Role of Blimp-1 in programing Th effector cells into IL-10 producers.
Authors: Neumann C, Heinrich F, Neumann K, Junghans V, Mashreghi M, Ahlers J, Janke M, Rudolph C, Mockel-Tenbrinck N, Kuhl A, Heimesaat M, Esser C, Im S, Radbruch A, Rutz S, Scheffold A
J Exp Med, 2014-07-29;211(9):1807-19.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The alarmin IL-33 promotes regulatory T-cell function in the intestine.
Authors: Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert E, Pott J, Griseri T, Bollrath J, Hegazy A, Harrison O, Owens B, Lohning M, Belkaid Y, Fallon P, Powrie F
Nature, 2014-07-16;513(7519):564-8.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of T cell motility in vitro and in vivo by LPA and LPA2.
Authors: Knowlden S, Capece T, Popovic M, Chapman T, Rezaee F, Kim M, Georas S
PLoS ONE, 2014-07-08;9(7):e101655.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Brain glycolipids suppress T helper cells and inhibit autoimmune demyelination.
Authors: Mycko M, Sliwinska B, Cichalewska M, Cwiklinska H, Raine C, Selmaj K
J Neurosci, 2014-06-18;34(25):8646-58.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The inhibitory effect of IFN-gamma on protease HTRA1 expression in rheumatoid arthritis.
Authors: Hou Y, Lin H, Zhu L, Liu Z, Hu F, Shi J, Yang T, Shi X, Guo H, Tan X, Zhang L, Wang Q, Li Z, Zhao Y
J Immunol, 2014-06-06;193(1):130-8.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
miR-20b suppresses Th17 differentiation and the pathogenesis of experimental autoimmune encephalomyelitis by targeting RORgammat and STAT3.
Authors: Zhu E, Wang X, Zheng B, Wang Q, Hao J, Chen S, Zhao Q, Zhao L, Wu Z, Yin Z
J Immunol, 2014-05-19;192(12):5599-609.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Elongated TCR alpha chain CDR3 favors an altered CD4 cytokine profile.
Authors: Reynolds C, Chong D, Raynsford E, Quigley K, Kelly D, Llewellyn-Hughes J, Altmann D, Boyton R
BMC Biol, 2014-05-09;12(0):32.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-21 induces IL-22 production in CD4+ T cells.
Authors: Yeste A, Mascanfroni I, Nadeau M, Burns E, Tukpah A, Santiago A, Wu C, Patel B, Kumar D, Quintana F
Nat Commun, 2014-05-06;5(0):3753.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of experimental autoimmune encephalomyelitis by TPL-2 kinase.
Authors: Sriskantharajah S, Guckel E, Tsakiri N, Kierdorf K, Brender C, Ben-Addi A, Veldhoen M, Tsichlis P, Stockinger B, O'Garra A, Prinz M, Kollias G, Ley S
J Immunol, 2014-03-17;192(8):3518-29.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Differential IL-10 production by DCs determines the distinct adjuvant effects of LPS and PTX in EAE induction.
Authors: Zhou H, Wang Y, Lian Q, Yang B, Ma Y, Wu X, Sun S, Liu Y, Sun B
Eur J Immunol, 2014-03-07;44(5):1352-62.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
CD49a promotes T-cell-mediated hepatitis by driving T helper 1 cytokine and interleukin-17 production.
Authors: Chen Y, Peng H, Chen Y, Wei H, Sun R, Tian Z
Immunology, 2014-03-01;141(3):388-400.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-gamma-producing T helper 17 cells.
Authors: Wang Y, Godec J, Ben-Aissa K, Cui K, Zhao K, Pucsek A, Lee Y, Weaver C, Yagi R, Lazarevic V
Immunity, 2014-02-13;40(3):355-66.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Excess IL-1 signaling enhances the development of Th17 cells by downregulating TGF-beta-induced Foxp3 expression.
Authors: Ikeda S, Saijo S, Murayama M, Shimizu K, Akitsu A, Iwakura Y
J Immunol, 2014-01-15;192(4):1449-58.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection.
Authors: Guo X, Qiu J, Tu T, Yang X, Deng L, Anders R, Zhou L, Fu Y
Immunity, 2014-01-09;40(1):25-39.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Dual-specificity phosphatase 14 (DUSP14/MKP6) negatively regulates TCR signaling by inhibiting TAB1 activation.
Authors: Yang C, Li J, Chiu L, Lan J, Chen D, Chuang H, Huang C, Tan T
J Immunol, 2014-01-08;192(4):1547-57.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
B cells promote induction of experimental autoimmune encephalomyelitis by facilitating reactivation of T cells in the central nervous system.
Authors: Pierson E, Stromnes I, Goverman J
J Immunol, 2013-12-23;192(3):929-39.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Janus kinase 1/3 signaling pathways are key initiators of TH2 differentiation and lung allergic responses.
Authors: Ashino S, Takeda K, Li H, Taylor V, Joetham A, Pine P, Gelfand E
J Allergy Clin Immunol, 2013-12-22;133(4):1162-74.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Therapeutic efficacy of suppressing the Jak/STAT pathway in multiple models of experimental autoimmune encephalomyelitis.
Authors: Liu Y, Holdbrooks A, De Sarno P, Rowse A, Yanagisawa L, McFarland B, Harrington L, Raman C, Sabbaj S, Benveniste E, Qin H
J Immunol, 2013-12-09;192(1):59-72.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Th1 polarization of T cells injected into the cerebrospinal fluid induces brain immunosurveillance.
Authors: Fisher Y, Strominger I, Biton S, Nemirovsky A, Baron R, Monsonego A
J Immunol, 2013-12-04;192(1):92-102.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Posttranscriptional gene regulation of IL-17 by the RNA-binding protein HuR is required for initiation of experimental autoimmune encephalomyelitis.
Authors: Chen J, Cascio J, Magee J, Techasintana P, Gubin M, Dahm G, Calaluce R, Yu S, Atasoy U
J Immunol, 2013-10-28;191(11):5441-50.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39.
Authors: Mascanfroni, Ivan D, Yeste, Ada, Vieira, Silvio M, Burns, Evan J, Patel, Bonny, Sloma, Ido, Wu, Yan, Mayo, Lior, Ben-Hamo, Rotem, Efroni, Sol, Kuchroo, Vijay K, Robson, Simon C, Quintana, Francisc
Nat Immunol, 2013-09-01;14(10):1054-63.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-23 protection against Plasmodium berghei infection in mice is partially dependent on IL-17 from macrophages.
Authors: Ishida H, Imai T, Suzue K, Hirai M, Taniguchi T, Yoshimura A, Iwakura Y, Okada H, Suzuki T, Shimokawa C, Hisaeda H
Eur J Immunol, 2013-08-19;43(10):2696-706.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
ERK differentially regulates Th17- and Treg-cell development and contributes to the pathogenesis of colitis.
Authors: Liu H, Yao S, Dann S, Qin H, Elson C, Cong Y
Eur J Immunol, 2013-06-03;43(7):1716-26.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation.
Authors: Yanaba K, Kamata M, Ishiura N, Shibata S, Asano Y, Tada Y, Sugaya M, Kadono T, Tedder T, Sato S
J Leukoc Biol, 2013-04-29;94(4):563-73.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-22-producing RORgammat-dependent innate lymphoid cells play a novel protective role in murine acute hepatitis.
Authors: Matsumoto A, Kanai T, Mikami Y, Chu P, Nakamoto N, Ebinuma H, Saito H, Sato T, Yagita H, Hibi T
PLoS ONE, 2013-04-23;8(4):e62853.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Autoreactive Th1 cells activate monocytes to support regional Th17 responses in inflammatory arthritis.
Authors: Simons D, Oh S, Kropf E, Aitken M, Garcia V, Basehoar A, Caton A
J Immunol, 2013-02-18;190(7):3134-41.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Laminin-411 Is a Vascular Ligand for MCAM and Facilitates TH17 Cell Entry into the CNS.
Authors: Flanagan K, Fitzgerald K, Baker J
PLoS ONE, 2012-07-06;7(7):e40443.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of innate CD8+ T-cell activation mediated by cytokines.
Proc Natl Acad Sci U S A, 2012-06-04;109(25):9971-6.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues.
Authors: Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D
J. Immunol., 2012-04-13;188(10):4866-75.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Difference in Th1 and Th17 lymphocyte adhesion to endothelium.
Authors: Alcaide P, Maganto-Garcia E, Newton G, Travers R, Croce KJ, Bu DX, Luscinskas FW, Lichtman AH
J. Immunol., 2012-01-04;188(3):1421-30.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus.
Authors: Monticelli LA, Sonnenberg GF, Abt MC
Nat. Immunol., 2011-11-01;12(11):1045-54.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus.
Authors: Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, Chan YR, Ouyang W, Brown GD, Weaver CT, Steele C
Infect. Immun., 2011-10-28;80(1):410-7.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-23-mediated inflammation in Pseudomonas aeruginosa pulmonary infection.
Authors: Dubin PJ, Martz A, Eisenstatt JR, Fox MD, Logar A, Kolls JK
Infect. Immun., 2011-10-24;80(1):398-409.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Preclinical Evaluation of Local JAK1 and JAK2 Inhibition in Cutaneous Inflammation.
Authors: Fridman JS, Scherle PA, Collins R, Burn T, Neilan CL, Hertel D, Contel N, Haley P, Thomas B, Shi J, Collier P, Rodgers JD, Shepard S, Metcalf B, Hollis G, Newton RC, Yeleswaram S, Friedman SM, Vaddi K
J. Invest. Dermatol., 2011-06-16;131(9):1838-44.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance.
Authors: Breslow JM, Meissler JJ, Hartzell RR
Infect. Immun., 2011-05-16;79(8):3317-27.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy.
Authors: Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, Lasarte JJ, Matsuzaki G, Ikuta K, Ryffel B, Benlagha K, Tesniere A, Ibrahim N, Dechanet-Merville J, Chaput N, Smyth MJ, Kroemer G, Zitvogel L
J. Exp. Med., 2011-03-07;208(3):491-503.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Distinct Roles of IL-23 and IL-17 in the Development of Psoriasis-Like Lesions in a Mouse Model.
Authors: Nakajima K, Kanda T, Takaishi M, Shiga T, Miyoshi K, Nakajima H, Kamijima R, Tarutani M, Benson JM, Elloso MM, Gutshall LL, Naso MF, Iwakura Y, DiGiovanni J, Sano S
J. Immunol., 2011-02-23;186(7):4481-9.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota.
Authors: Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, Di Santo JP, Eberl G
Nat. Immunol., 2011-02-20;12(4):320-6.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens.
Authors: DePaolo RW, Abadie V, Tang F
Nature, 2011-02-09;471(7337):220-4.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus.
Authors: Kayamuro H, Yoshioka Y, Abe Y, Arita S, Katayama K, Nomura T, Yoshikawa T, Kubota-Koketsu R, Ikuta K, Okamoto S, Mori Y, Kunisawa J, Kiyono H, Itoh N, Nagano K, Kamada H, Tsutsumi Y, Tsunoda S
J. Virol., 2010-09-29;84(24):12703-12.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Naive and activated T cells display differential responsiveness to TL1A that affects Th17 generation, maintenance, and proliferation.
Authors: Jones GW, Stumhofer JS, Foster T, Twohig JP, Hertzog P, Topley N, Williams AS, Hunter CA, Jenkins BJ, Wang EC, Jones SA
FASEB J., 2010-09-08;25(1):409-19.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-22 defines a novel immune pathway of antifungal resistance.
Authors: De Luca A, Zelante T, D'Angelo C
Mucosal Immunol, 2010-05-05;3(4):361-73.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice.
Authors: Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS
J. Clin. Invest., 2010-04-01;120(5):1762-73.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-23 is required for protection against systemic infection with Listeria monocytogenes.
Authors: Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE
J. Immunol., 2009-12-15;183(12):8026-34.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFNgamma production.
Authors: Lemos HP, Grespan R, Vieira SM, Cunha TM, Verri WA, Fernandes KS, Souto FO, McInnes IB, Ferreira SH, Liew FY, Cunha FQ
Proc. Natl. Acad. Sci. U.S.A., 2009-03-16;106(14):5954-9.
Species: Mouse
Sample Types: In Vivo, N/A
Applications: ELISA (Standard), In Vivo -
Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge.
Authors: Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW
J. Immunol., 2009-03-15;182(6):3469-81.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice.
Authors: McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK
J. Immunol., 2008-09-15;181(6):4089-97.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin.
Authors: Shinohara ML, Kim JH, Garcia VA, Cantor H
Immunity, 2008-07-18;29(1):68-78.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice.
Authors: Guo B, Chang EY, Cheng G
J. Clin. Invest., 2008-05-01;118(5):1680-90.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice.
Authors: Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K
J. Immunol., 2007-12-15;179(12):8274-9.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells.
Authors: Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A
Nat. Immunol., 2007-11-11;8(12):1372-9.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance.
Authors: Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L
Eur. J. Immunol., 2007-10-01;37(10):2695-706.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4.
Authors: Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M
Nat. Immunol., 2007-08-05;8(9):958-66.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Involvement of mast cells in IL-12/23 p40 production is essential for survival from polymicrobial infections.
Authors: Nakano N, Nishiyama C, Kanada S, Niwa Y, Shimokawa N, Ushio H, Nishiyama M, Okumura K, Ogawa H
Blood, 2007-02-08;109(11):4846-55.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Functional relevance of the IL-23-IL-17 axis in lungs in vivo.
Authors: Ivanov S, Bozinovski S, Bossios A, Valadi H, Vlahos R, Malmhall C, Sjostrand M, Kolls JK, Anderson GP, Linden A
Am. J. Respir. Cell Mol. Biol., 2006-12-01;36(4):442-51.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells.
Authors: Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK
Nature, 2006-04-30;441(7090):235-8.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages.
Authors: Harrington, Laurie E, Hatton, Robin D, Mangan, Paul R, Turner, Henriett, Murphy, Theresa, Murphy, Kenneth, Weaver, Casey T
Nat Immunol, 2005-10-02;6(11):1123-32.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay
FAQs
-
Should I use Recombinant Mouse IL-23 Protein formulated with carrier BSA (Catalog # 1887-ML) or the Recombinant Mouse IL-23 Protein, CF (Catalog # 1887-ML/CF) that is carrier-free (no BSA) for my in vivo experiments?
For in vivo aplications, we would recommend using the carrier-free Mouse IL-23 Protein (Catalog # 1887-ML/CF). The injection of a carrier protein such as BSA can cause side effects and sometimes death to the experimental animal. For more information regarding use of the protein for in vivo applications, we would recommend consulting the Citations tab on the product-specific page.
Reviews for Recombinant Mouse IL-23 Protein
Average Rating: 4.8 (Based on 6 Reviews)
Have you used Recombinant Mouse IL-23 Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
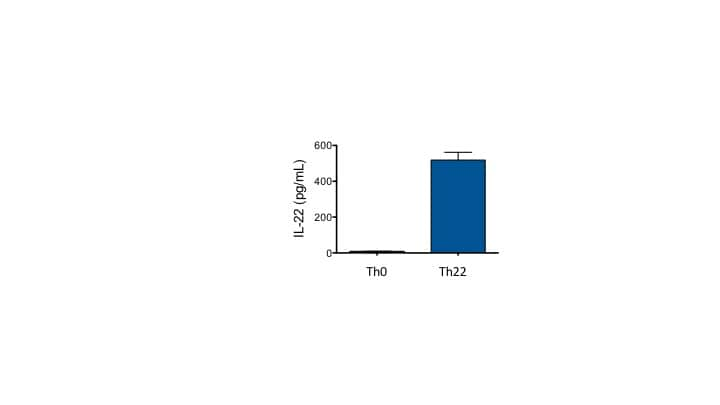
Reason for Rating: It works for Th17 differentiation
T cells were differentiated in the presence of IL-6, IL-1beta, IL-23, anti-IFNg, and anti-IL-4 for 4 days. IL-22 was measured in the culture supernatant.