Recombinant S. cerevisiae SUMO Protease ULP1 His Protein, CF New
Recombinant S. cerevisiae SUMO Protease ULP1 His Protein, CF Summary
- R&D Systems E. coli-derived Recombinant S. cerevisiae SUMO Protease ULP1 His Protein (11697-SO)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Product Specifications
Leu403-Lys621, with an N-terminal Met and 6-His tag
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
11697-SO
| Formulation | Supplied as a 0.2 μm filtered solution in Tris, NaCl, DTT and Glycerol. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Assay Procedure
- Assay Buffer: 50 mM Tris, 5 mM DTT, pH 8.0
- Recombinant Yeast SUMO Protease ULP1 His-tag (SUMO Protease ULP1) (Catalog # 11697-SO)
- Recombinant SUMO-GFP Protease Substrate (rSUMO-GFP) (Catalog # 11698-SO)
- 15% SDS-PAGE gel
- Reducing Sample Buffer
- Gel Staining Reagent
- Prepare a curve of SUMO Protease ULP1 by diluting SUMO Protease ULP1 to 20, 1.25, 0.312, 0.156, 0.078, 0.039 and 0.00977 µg/mL in Assay Buffer.
- Dilute rSUMO-GFP to 200 µg/mL in Assay Buffer.
- Combine 20 µL of each SUMO Protease ULP1 curve dilution and 20 µL of 200 µg/mL rSUMO-GFP. Prepare a Control by combining 20 µL of 200 µg/mL rSUMO-GFP and 20 µL Assay Buffer.
- Incubate reaction mixtures and control at room temperature for 2 hours.
- Stop the reactions by combining equal volumes of reaction mixture (including control) and Reducing SDS-PAGE Sample Buffer. Heat all samples at 95 °C for 3 minutes.
- Load 20 µL of each stopped reaction per lane on a 15% SDS-PAGE gel and perform electrophoresis.
- Stain gel and analyze the % cleavage of rSUMO-GFP using densitometry for each SUMO Protease ULP1 curve dilution.
- Determine the DC50 by plotting % cleavage vs SUMO Protease ULP1 concentration (ng) using 4-PL fitting.
- SUMO Protease ULP1: 100, 6.25, 1.56, 0.78, 0.39, 0.195, 0.0488, and 0 ng
- rSUMO-GFP: 1 µg
Scientific Data
 View Larger
View Larger
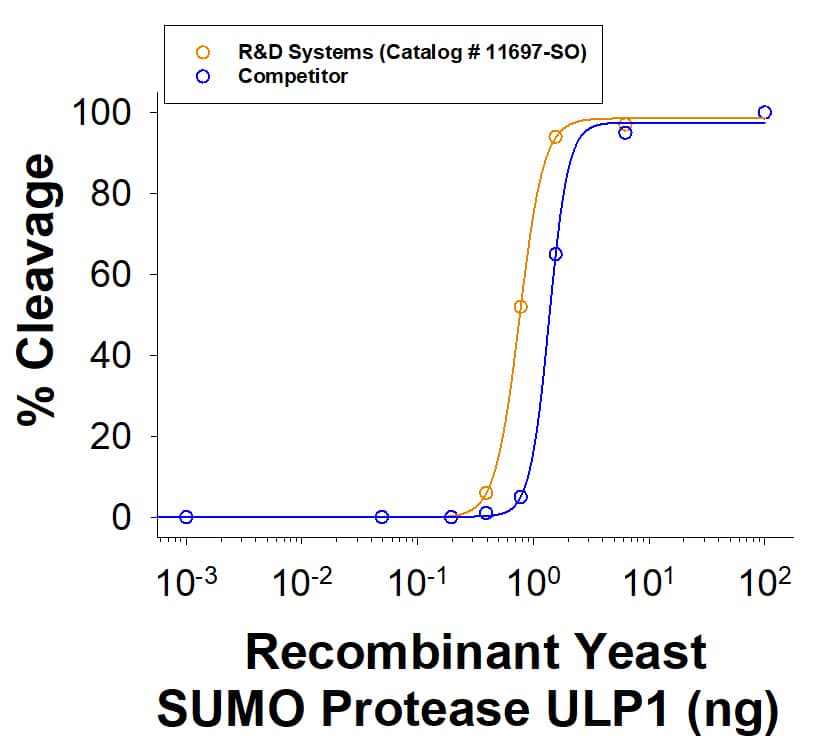
Recombinant Yeast SUMO Protease ULP1 His-tag activity is measured by its ability to cleave SUMO-GFP Protease Substrate (11698-SO). The R&D Systems protein has higher activity than competitor protein in direct comparison.
 View Larger
View Larger
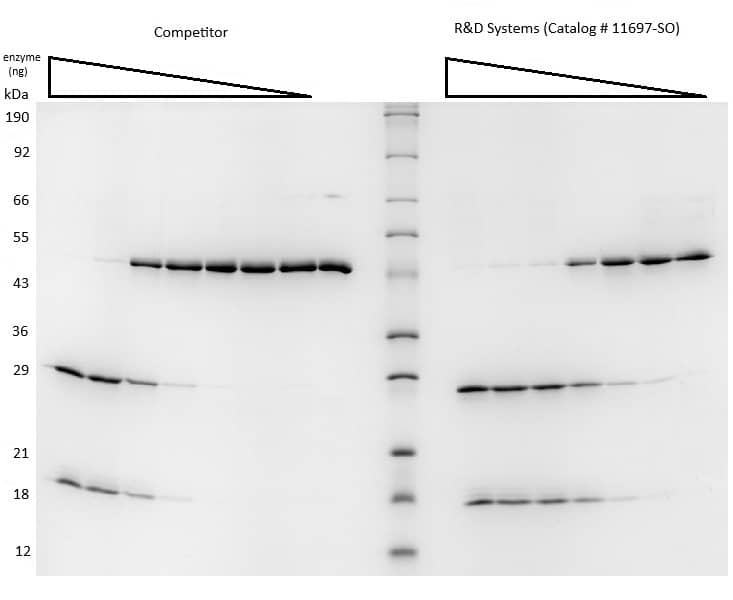
Recombinant Yeast SUMO Protease ULP1 His-tag Protein (Catalog # 11697-SO) from R&D Systems and a competitor product are able to cleave Recombinant SUMO-GFP Protease Substrate (~47 kDa, 11698-SO) per insert assay conditions. Cleavage is detected with SDS-PAGE under reducing (R) conditions and visualized by Coomassie® Blue staining. The enzyme from R&D Systems offers a better value than the competition.
 View Larger
View Larger
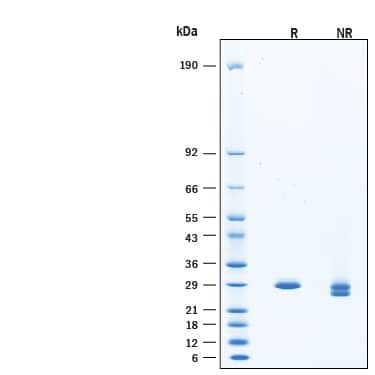
2 μg/lane of Recombinant Yeast SUMO Protease ULP1 His-tag Protein (Catalog # 11697-SO) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 27-33 kDa, under reducing conditions.
Reconstitution Calculator
Background: SUMO Protease ULP1
Recombinant Yeast SUMO Protease ULP1 (Ubiquitin-like-specific protease 1), commonly known as SUMO protease 1, is essential in the processing and deconjugation of a small ubiquitin-like modifier (SUMO), SMT3, from targeted proteins in the SUMO pathway of yeast where it plays an essential role in the cell cycle (1, 2). ULP1 is a 621 amino acid protein from the peptidase C48 family of cysteine proteases and contains a conserved C-terminal protease fold domain and a weakly conserved N-terminal domain (1, 3). The catalytic site with a conserved cysteine protease structure and catalytic triad is located in a shallow and narrow cleft that creates limited access to the active site through the constricted hydrophobic tunnel and confers high specificity to the enzyme (3). ULP1 cleaves the C-terminus of SUMO to a mature form after a glycine-glycine pair. Use of SUMO as a fusion for recombinant proteins with subsequent processing by ULP1 in E. coli was explored due to the demand to find improved methods for recombinant production of proteins for use in academia and industry (4). SUMO as a fusion partner was found to enhance expression in E. coli for a diverse population of protein targets and unlike other common fusion partners SUMO could serve as a chaperone for correct folding such that the target would remain soluble after the SUMO protein was removed (4). In addition, ULP1 was found to be capable of cleaving with high efficiency a range of targets specifically at the SUMO site under a broad variety of reaction conditions (5, 6). Use of SUMO fusion with ULP1 cleavage is a tool that allows for successful recombinant protein production with a native N-terminal sequence (5) providing significant benefits over other fusion and cleavage methods being used (4,7-10). Use of recombinant SUMO fusions with subsequent ULP1 cleavage is a beneficial tool for recombinant protein production in both academic research and industrial biotechnology (4, 5, 11).
- Li, S.J and M. Hochstrasser (1999) Nature 398:246.
- Li, S.J and M. Hochstrasser (2000) Mol Cell Biol. 20:2367.
- Mossessova, E. and C.D. Lima (2000) Mol. Cell 5:865.
- Malakhov, M.P. et al. (2004) J. Struct. Funct. Genomics 5:75.
- Marblestone, J.G. et al. (2006) Protein Sci. 15:182.
- Satakarni, M. and R. Curtis (2011) Protein Expr. Purif. 78:113.
- Baker, R.T. (1996) Curr. Opin. Biotechnol. 7:541.
- Jonasson, P. et al. (2002) Biotechnol. Appl. Biochem. 35:91.
- Butt, T.R. et al. (2005) Protein Expr. Purif. 43:1.
- Peroutka, R.J. et al. (2011) Methods Mol. Biol. 705:15.
- Wang, Z. et al. (2010) Protein Expr. Purif. 73:203.
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant S. cerevisiae SUMO Protease ULP1 His Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant S. cerevisiae SUMO Protease ULP1 His Protein, CF and earn rewards!
Have you used Recombinant S. cerevisiae SUMO Protease ULP1 His Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
