Key Product Details
Species Reactivity
Applications
Label
Antibody Source
Product Specifications
Immunogen
Glu24-Lys631
Accession # O00206
Specificity
Clonality
Host
Isotype
Applications for TLR4 Antibody [DyLight 405]
CyTOF-ready
Flow Cytometry
Immunocytochemistry
Immunohistochemistry
Neutralization
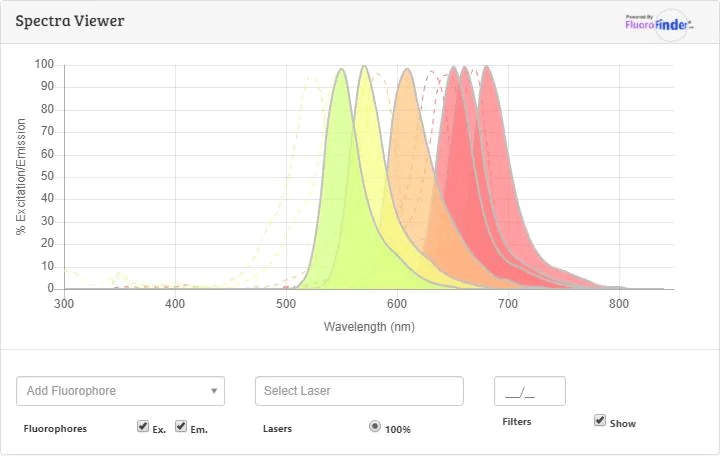
Spectra Viewer
Plan Your Experiments
Use our spectra viewer to interactively plan your experiments, assessing multiplexing options. View the excitation and emission spectra for our fluorescent dye range and other commonly used dyes.
Spectra ViewerFlow Cytometry Panel Builder
Bio-Techne Knows Flow Cytometry
Save time and reduce costly mistakes by quickly finding compatible reagents using the Panel Builder Tool.
Advanced Features
- Spectra Viewer - Custom analysis of spectra from multiple fluorochromes
- Spillover Popups - Visualize the spectra of individual fluorochromes
- Antigen Density Selector - Match fluorochrome brightness with antigen density
Formulation, Preparation, and Storage
Purification
Formulation
Preservative
Concentration
Shipping
Stability & Storage
Background: TLR4
TLR4 signaling occurs through two distinct pathways: The MyD88 (myeloid differentiation primary response gene 88)-dependent pathway and the MyD88-independent (TRIF-dependent, TIR domain-containing adaptor inducing IFN-beta) pathway (3, 5-7). The MyD88-dependent pathway occurs mainly at the plasma membrane and involves the binding of MyD88-adaptor-like (MAL) protein followed by a signaling cascade that results in the activation of transcription factors including nuclear factor-kappaB (NF-kappaB) that promote the secretion of inflammatory molecules and increased phagocytosis (5-7). Conversely, the MyD88-independent pathway occurs after TLR4-MD2 complex internalization in the endosomal compartment. This pathway involves the binding of adapter proteins TRIF and TRIF-related adaptor molecule (TRAM), a signaling activation cascade resulting in IFN regulatory factor 3 (IRF3) translocation into the nucleus, and secretion of interferon-beta (INF-beta) genes and increased phagocytosis (5-7).
Given its expression on immune-related cells and its role in inflammation, TLR4 activation can contribute to various diseases (6-8). For instance, several studies have found that TLR4 activation is associated with neurodegeneration and several central nervous system (CNS) pathologies, including Alzheimer's disease, Parkinson's disease, and Huntington's disease (6, 7). Furthermore, TLR4 mutations have been shown to lead to higher rates of infections and increased susceptibility to sepsis (7-8). One potential therapeutic approach aimed at targeting TLR4 and neuroinflammation is polyphenolic compounds which include flavonoids and phenolic acids and alcohols (8).
Alternative names for TLR4 includes 76B357.1, ARMD10, CD284 antigen, CD284, EC 3.2.2.6, homolog of Drosophila toll, hToll, toll like receptor 4 protein, TOLL, toll-like receptor 4.
References
1. Vaure, C., & Liu, Y. (2014). A comparative review of toll-like receptor 4 expression and functionality in different animal species. Frontiers in immunology. https://doi.org/10.3389/fimmu.2014.00316
2. Park, B. S., & Lee, J. O. (2013). Recognition of lipopolysaccharide pattern by TLR4 complexes. Experimental & molecular medicine. https://doi.org/10.1038/emm.2013.97
3. Krishnan, J., Anwar, M.A., & Choi, S. (2016) TLR4 (Toll-Like Receptor 4). In: Choi S. (eds) Encyclopedia of Signaling Molecules. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6438-9_592-1
4. Botos, I., Segal, D. M., & Davies, D. R. (2011). The structural biology of Toll-like receptors. Structure. https://doi.org/10.1016/j.str.2011.02.004
5. Lu, Y. C., Yeh, W. C., & Ohashi, P. S. (2008). LPS/TLR4 signal transduction pathway. Cytokine. https://doi.org/10.1016/j.cyto.2008.01.006
6. Leitner, G. R., Wenzel, T. J., Marshall, N., Gates, E. J., & Klegeris, A. (2019). Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Expert opinion on therapeutic targets. https://doi.org/10.1080/14728222.2019.1676416
7. Molteni, M., Gemma, S., & Rossetti, C. (2016). The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediators of inflammation. https://doi.org/10.1155/2016/6978936
8. Rahimifard, M., Maqbool, F., Moeini-Nodeh, S., Niaz, K., Abdollahi, M., Braidy, N., Nabavi, S. M., & Nabavi, S. F. (2017). Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing research reviews. https://doi.org/10.1016/j.arr.2017.02.004
Long Name
Alternate Names
Gene Symbol
Additional TLR4 Products
Product Documents for TLR4 Antibody [DyLight 405]
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for TLR4 Antibody [DyLight 405]
DyLight (R) is a trademark of Thermo Fisher Scientific Inc. and its subsidiaries.
This product is for research use only and is not approved for use in humans or in clinical diagnosis. Primary Antibodies are guaranteed for 1 year from date of receipt.
Customer Reviews for TLR4 Antibody [DyLight 405]
There are currently no reviews for this product. Be the first to review TLR4 Antibody [DyLight 405] and earn rewards!
Have you used TLR4 Antibody [DyLight 405]?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Protocols
Find general support by application which include: protocols, troubleshooting, illustrated assays, videos and webinars.
- 7-Amino Actinomycin D (7-AAD) Cell Viability Flow Cytometry Protocol
- Antigen Retrieval Protocol (PIER)
- Antigen Retrieval for Frozen Sections Protocol
- Appropriate Fixation of IHC/ICC Samples
- Cellular Response to Hypoxia Protocols
- Chromogenic IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Chromogenic Immunohistochemistry Staining of Frozen Tissue
- Detection & Visualization of Antibody Binding
- Extracellular Membrane Flow Cytometry Protocol
- Flow Cytometry Protocol for Cell Surface Markers
- Flow Cytometry Protocol for Staining Membrane Associated Proteins
- Flow Cytometry Staining Protocols
- Flow Cytometry Troubleshooting Guide
- Fluorescent IHC Staining of Frozen Tissue Protocol
- Graphic Protocol for Heat-induced Epitope Retrieval
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Graphic Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- ICC Cell Smear Protocol for Suspension Cells
- ICC Immunocytochemistry Protocol Videos
- ICC for Adherent Cells
- IHC Sample Preparation (Frozen sections vs Paraffin)
- Immunocytochemistry (ICC) Protocol
- Immunocytochemistry Troubleshooting
- Immunofluorescence of Organoids Embedded in Cultrex Basement Membrane Extract
- Immunofluorescent IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Immunohistochemistry (IHC) and Immunocytochemistry (ICC) Protocols
- Immunohistochemistry Frozen Troubleshooting
- Immunohistochemistry Paraffin Troubleshooting
- Intracellular Flow Cytometry Protocol Using Alcohol (Methanol)
- Intracellular Flow Cytometry Protocol Using Detergents
- Intracellular Nuclear Staining Flow Cytometry Protocol Using Detergents
- Intracellular Staining Flow Cytometry Protocol Using Alcohol Permeabilization
- Intracellular Staining Flow Cytometry Protocol Using Detergents to Permeabilize Cells
- Preparing Samples for IHC/ICC Experiments
- Preventing Non-Specific Staining (Non-Specific Binding)
- Primary Antibody Selection & Optimization
- Propidium Iodide Cell Viability Flow Cytometry Protocol
- Protocol for Heat-Induced Epitope Retrieval (HIER)
- Protocol for Making a 4% Formaldehyde Solution in PBS
- Protocol for VisUCyte™ HRP Polymer Detection Reagent
- Protocol for the Characterization of Human Th22 Cells
- Protocol for the Characterization of Human Th9 Cells
- Protocol for the Fluorescent ICC Staining of Cell Smears - Graphic
- Protocol for the Fluorescent ICC Staining of Cultured Cells on Coverslips - Graphic
- Protocol for the Preparation & Fixation of Cells on Coverslips
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections - Graphic
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections - Graphic
- Protocol for the Preparation and Fluorescent ICC Staining of Cells on Coverslips
- Protocol for the Preparation and Fluorescent ICC Staining of Non-adherent Cells
- Protocol for the Preparation and Fluorescent ICC Staining of Stem Cells on Coverslips
- Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- Protocol for the Preparation of a Cell Smear for Non-adherent Cell ICC - Graphic
- Protocol: Annexin V and PI Staining by Flow Cytometry
- Protocol: Annexin V and PI Staining for Apoptosis by Flow Cytometry
- TUNEL and Active Caspase-3 Detection by IHC/ICC Protocol
- The Importance of IHC/ICC Controls
- Troubleshooting Guide: Fluorokine Flow Cytometry Kits
- Troubleshooting Guide: Immunohistochemistry
- View all Protocols, Troubleshooting, Illustrated assays and Webinars