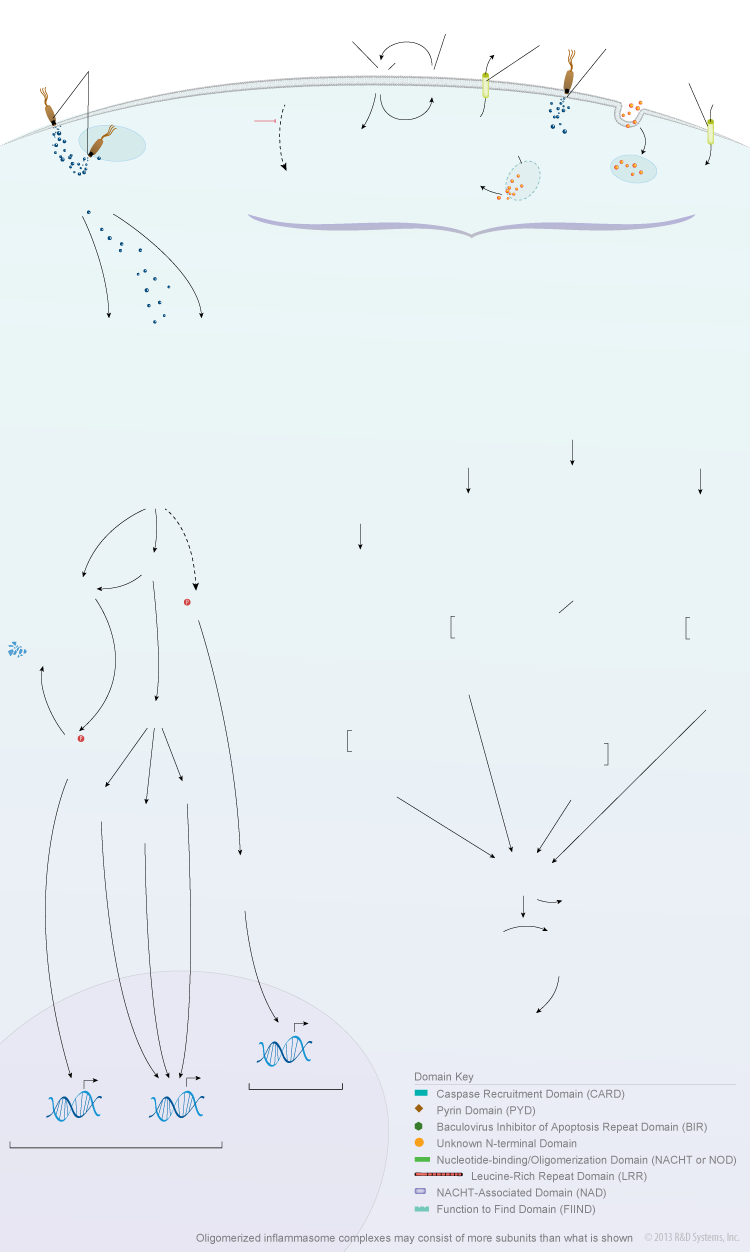
NOD-like Receptor Signaling Pathways
Secretion Systems
or Endosome
PGN Components
cIAP-2
cIAP-2
or IRF7
Homodimer
IFN-inducible genes
Receptor
Secretion Systems
Activators
and Pro-IL-18
Oligomerization
NALP1
NALP3
IPAF
IPAF
| NOD1 |
|---|
| Select Agonist(s) |
| Meso-diaminopimelic acid |
| NOD2 |
|---|
| Select Agonist(s) |
| Muramyl dipeptide |
| NLRP1/NALP1 |
|---|
| Select Agonist(s) |
| Bacillus anthracis lethal toxin |
| Muramyl dipeptide |
| NLRP3/NALP3 |
|---|
| Select Agonist(s) |
| Adenoviral DNA |
| Bacterial RNA |
| Influenza and Sendai virus RNA |
| Lipopolysaccharide |
| Pore-forming toxins: Hemolysins, Nigericin, |
| Pneumolysin |
| Exogenous compounds: Silica, Asbestos, Alum |
| Danger signals: Extracellular ATP, extracellular NAD+, |
| beta-amyloid and particulates such as calcium pyrophosphate dihydrate and monosodium urate |
| Candida albicans |
| Saccharomyces cerevisiae |
| Klebsiella pneumoniae |
| Mycobacterium tuberculosis |
| Salmonella typhimurium |
| Schistosoma mansoni |
| NLRP7/NALP7 |
|---|
| Select Agonist(s) |
| Acylated microbial lipopeptides |
| NLRP12/NALP12 |
|---|
| Select Agonist(s) |
| Yersinia pestis |
| NLRC4/IPAF |
|---|
| Select Agonist(s) |
| Legionella pneumophila |
| Pseudomonas aeruginosa |
| Salmonella typhimurium |
| Shigella flexneri |
| NAIP |
| Select Agonist(s) |
| Flagellin (Naip5) |
| Components of the bacterial type III secretion apparatus (Naip2) |
Featured Literature
Inflammasomes: Intracellular Regulators of Pathogen Recognition, Host Defense, and Inflammation Poster
Overview of NOD-like Receptors
Nucleotide-binding, oligomerization domain (NOD)-like receptors (NLRs) are a family of cytosolic proteins that play an important role in inflammation and immunity. The NLR family consists of twenty-two human proteins and at least thirty-four mouse proteins that contain a central nucleotide-binding, oligomerization domain (NOD or NACHT), a variable number of C-terminal leucine-rich repeats, and an N-terminal protein interaction domain. Similar to the leucine-rich repeat-containing toll-like receptors (TLRs), multiple NLRs function as pattern recognition receptors. These NLRs are responsible for detecting specific pathogen-associated molecules or host-derived damage signals in the cytosol and initiating the innate immune response. NOD1/NLRC1 and NOD2/NLRC2 are two pattern recognition receptors that are activated by specific components of bacterial peptidoglycan. Their activation triggers signaling pathways that drive the NF-kappa B-/AP-1-dependent expression of pro-inflammatory cytokines and the IRF3- and/or IRF7-dependent expression of type I interferons. Additionally, activation of NOD1 and NOD2 can induce autophagy through an interaction with Autophagy-related 16-like 1 (ATG16L1). Although a clear connection between NOD1 and chronic inflammatory diseases has not been well-established, mutations in NOD2 have been linked to Crohn’s disease and Blau syndrome.
Other NLRs that function as pattern recognition receptors, including NLRP1, NLRP3, NLRP6, NLRP7, NLRP12, NLRC4/IPAF, and NAIP, oligomerize to form multiprotein inflammasome complexes following their activation by different pathogens or endogenous danger signals. Inflammasome oligomerization leads to the cleavage and activation of Caspase-1, which subsequently promotes the processing and secretion of IL-1 beta and IL-18, and can induce an inflammatory form of cell death known as pyroptosis. The NLRP1, NLRP3, and NLRC4/IPAF inflammasomes are the most fully characterized NLR-containing inflammasomes. They consist of a specific NLR protein, Apoptosis-associated Speck-like protein containing a CARD (ASC), Pro-Caspase-1, and inflammasome-specific proteins such as the Cardinal adaptor protein (NLRP3), Pro-Caspase-5 (NLRP1), or NAIP (NLRC4/IPAF). Inflammasome-mediated secretion of IL-1 beta and IL-18 is aimed at eliminating the infectious pathogen through the induction of secondary mediators and the recruitment of additional immune cells to the infection site and/or pyroptosis of the infected cells. While the inflammasome-mediated response is beneficial for the host, it must be tightly regulated as mutations that result in constitutive inflammasome activation have been linked to autoinflammatory disorders such as Familial Cold Auto-inflammatory Syndrome (FCAS), Familial Mediterranean Fever (FMF), Muckle-Wells Syndrome (MWS), and Chronic Infantile Neurologic Cutaneous and Articular Syndrome (CINCA). In addition, a number of other diseases such as inflammatory bowel disease, type 1 and type 2 diabetes, vitiligo, gout, and multiple sclerosis have also been suggested to be associated with inappropriate or chronic inflammasome activation.
In contrast to the NLRs involved in pathogen recognition, other members of the NLR family have been suggested to have anti-inflammatory effects due to their abilities to either negatively regulate NF-kappa B signaling or inhibit Caspase-1-mediated IL-1 beta secretion. Although these proteins have not been as widely investigated, preliminary studies suggest that NLRC3, NLRP2, NLRP4, NLRP7, NLRP10/PYNOD, NLRP12, and NLRX1 may have anti-inflammatory properties. In addition, there are two other unique NLRs, NLRC5 and Class II transactivator (CTIIA), that function as transcriptional regulators of MHC class I and MHC class II gene expression, respectively. Besides these activities, some NLR proteins may have additional immune modulatory functions. For example, NLRP10/PYNOD and NLRP12 have been shown to be involved in mediating dendritic cell migration and initiation of the adaptive immune response. Further research is necessary to determine whether other proteins belonging to the NLR family also have unique cell type-specific or context-dependent functions that play a role in regulating the immune response.
To learn more, please visit our NOD-like Receptors and the Inflammasome Research Area.
| Table 1. Antibodies for Detecting NOD-like Receptors - Products by Molecule | |||||
| NLRP1/NALP1 | NLRP2/NALP2 | NLRP3/NALP3 | NLRP6/NALP6 | NLRP10/Pynod/NALP10 | NOD1 |
| Table 2. Antibodies for Detecting Intracellular Molecules Involved in NOD-like Receptor Signaling - Products by Molecule | |||||
| ASC | CARD9 | Caspase-1 | cIAP-1/HIAP-2 | cIAP-2/HIAP-1 | ERK1 |
| ERK1/2 | ERK2 | I kappa B alpha | I kappa B beta | I kappa B epsilon | IKK-alpha |
| IKK-beta | IKK-gamma | IKK-epsilon | IL-1 beta | IL-18 | IRF3 |
| JNK | JNK1 | JNK2 | c-Jun | JunB | JunD |
| MKK3 | MKK3/MKK6 | MKK4 | MKK6 | MKK7 | NF kappa B1 |
| NF kappa B2 | p38 alpha | p38 beta | p38 gamma | p38 delta | c-Rel |
| RelA/NF kappa B p65 | RelB | TAB1 | TAK1 | ||
| Table 3. Select ELISAs for Quantifying Cytokines Secreted in Response to NOD-like Receptor Activation | |||||
| IFN-alpha | IFN-beta | IL-1 beta | IL-6 | IL-8/CXCL8 | TNF-alpha |
| Table 4. Small Molecule Inflammasome Inhibitors |
| Infllammasome Inhibitors |


