Human/Mouse/Rat Phospho-RSK1 (S380) Antibody
Human/Mouse/Rat Phospho-RSK1 (S380) Antibody Summary
Accession # Q15418
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
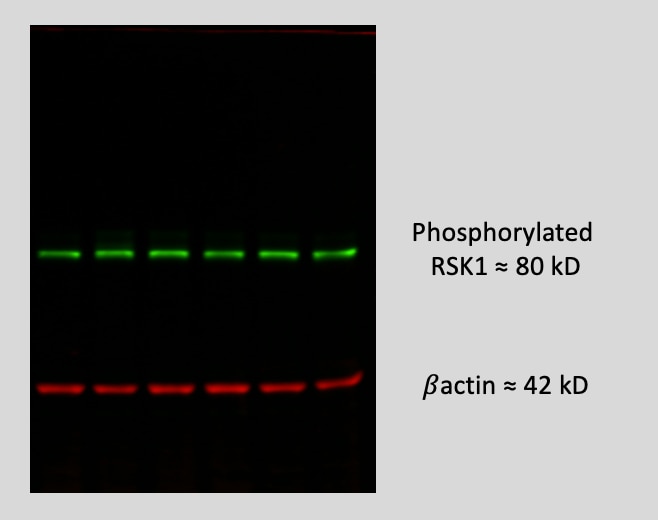
Detection of Human Phospho-RSK1 (S380) by Western Blot. Western blot shows lysates of Jurkat human acute T cell leukemia cell line and HeLa human cervical epithelial carcinoma cell line untreated (-) or treated (+) with 200 nM PMA or PMA and Ionomycin for 20 minutes. PVDF membrane was probed with 0.1 µg/mL of Rabbit Anti-Human/Mouse/Rat Phospho-RSK1 (S380) Monoclonal Antibody (Catalog # MAB79671) followed by HRP-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog # HAF008). A specific band was detected for Phospho-RSK1 (S380) at approximately 93 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
RSK1 in HeLa Human Cell Line. RSK1 was detected in immersion fixed HeLa human cervical epithelial carcinoma cell line, unstimulated (panels B and D) or stimulated with PMA (panels A and C), using Rabbit Anti-Human/Mouse/Rat Phospho-RSK1 (S380) Monoclonal Antibody (Catalog # MAB79671) at 25 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Rabbit IgG Secondary Antibody (red, upper panels; Catalog # NL004) and counterstained with DAPI (blue, lower panels). Specific staining was localized to plasma membrances and cytoplasm. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Detection of Human Phospho-RSK1 (S380) by Simple WesternTM. Simple Western lane view shows lysates of HeLa human cervical epithelial carcinoma cell line untreated (-) or treated (+) with 200 nM PMA for 20 minutes, and loaded at 0.2 mg/mL. A specific band was detected for Phospho-RSK1 (S380) at approximately 90 kDa (as indicated) using 1 μg/mL of Rabbit Anti-Human/Mouse/Rat Phospho-RSK1 (S380) Monoclonal Antibody (Catalog # MAB79671). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: RSK1
RSK1 (ribosomal S6 kinase 1), gene name RPS6KA1 (ribosomal protein S6 kinase alpha 1), also called p90RSK1 (90 kDa RSK1) or MAPKAPK 1a (MAPK-activated protein kinase 1a), is a widely expressed member of the RSK family of growth factor-regulated serine/threonine kinases. RSK proteins contain two non-identical kinase catalytic domains, and mediate activation of mitogen-activated kinase (MAPK) cascades and stimulation of cell proliferation and differentiation. Autophosphorylation on S380 allows binding of PDPK1, leading to further phosphorylation and full activation of RSK1 kinase activity. Human, mouse and rat RSK1 share 98% aa sequence identity, and are identical within the region of the peptide immunogen.
Product Datasheets
Citation for Human/Mouse/Rat Phospho-RSK1 (S380) Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
1 Citation: Showing 1 - 1
-
PRAS40 Connects Microenvironmental Stress Signaling to Exosome-Mediated Secretion
Authors: J Guo, P Jayaprakas, J Dan, P Wise, GB Jang, C Liang, M Chen, DT Woodley, M Fabbri, W Li
Mol. Cell. Biol., 2017-09-12;0(0):.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse/Rat Phospho-RSK1 (S380) Antibody
Average Rating: 5 (Based on 1 Review)
Have you used Human/Mouse/Rat Phospho-RSK1 (S380) Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: