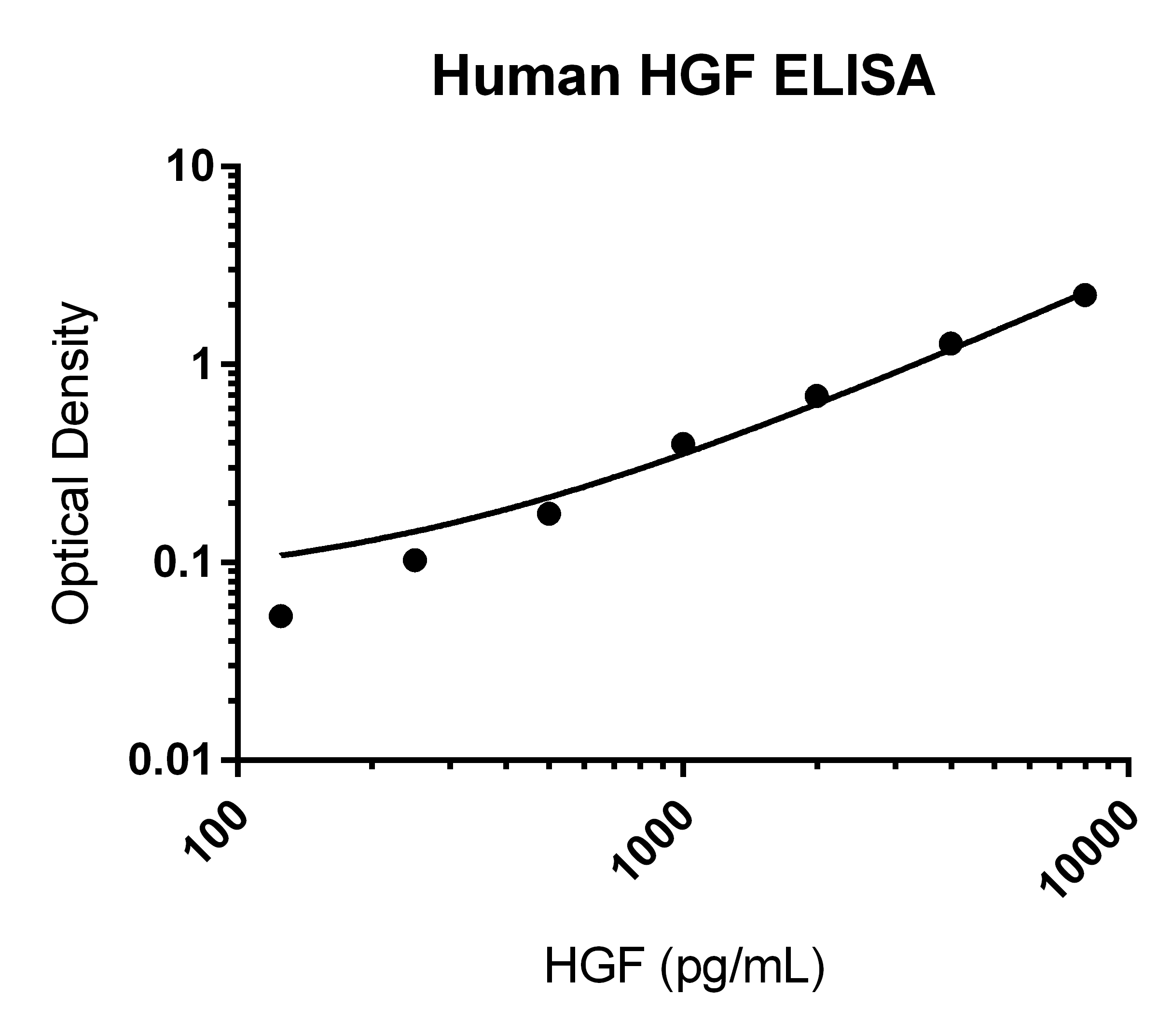
Human HGF Quantikine ELISA Kit Summary
Sample Values
Serum/Plasma - Samples from apparently healthy volunteers were evaluated for the presence of human HGF in this assay. No medical histories were available for the donors used in this study.| Sample Type | Mean (pg/mL) | Range (pg/mL) | Standard Deviation (pg/mL) |
| Serum (n=60) | 1257 | 671-1992 | 289 |
| EDTA plasma (n=60) | 787 | 469-113 | 136 |
| Citrate plasma (n=71) | 440 | 251-742 | 102 |
Cell Culture Supernates - Human peripheral blood mononuclear cells (5 x 106 cells/mL) were cultured in RPMI supplemented with 5% fetal calf serum, 50 μM beta -mercaptoethanol, 2 mM L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin sulfate. The cells were cultured unstimulated or stimulated with 10 μg/mL PHA for 1 and 5 days. Aliquots of the cell culture supernates were removed and assayed for levels of human HGF.
| Condition | Day 1 (pg/mL) | Day 5 (pg/mL) |
| Unstimulated | ND | 548 |
| Stimulated | 152 | 145 |
Product Summary
Precision
Cell Culture Supernates
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 425 | 991 | 3869 | 409 | 1058 | 4058 |
| Standard Deviation | 30.2 | 43.2 | 234 | 29.4 | 75.1 | 264 |
| CV% | 7.1 | 4.4 | 6 | 7.2 | 7.1 | 6.5 |
Serum, EDTA Plasma, Citrate Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 339 | 999 | 3759 | 363 | 967 | 3790 |
| Standard Deviation | 23.7 | 56.2 | 154 | 25.8 | 81.2 | 205 |
| CV% | 7 | 5.6 | 4.1 | 7.1 | 8.4 | 5.4 |
Recovery
The recovery of HGF spiked to three different levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=5) | 92 | 86-101 |
| Citrate Plasma (n=5) | 105 | 99-114 |
| EDTA Plasma (n=5) | 100 | 85-111 |
| Serum (n=5) | 99 | 91-108 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: HGF
HGF (Hepatocyte Growth Factor, Scatter Factor) induces the proliferation and migration of epithelial cells, hepatocytes, chondrocytes, keratinocytes, melanocytes, endothelial cells, and many tumor cells. During organogenesis and tissue repair, HGF promotes epithelial/endothelial morphogenesis by inducing cell scattering and branching tubulogenesis. It also supports insulin production by pancreatic beta cells, neuronal survival, and immune tolerance. HGF is secreted as a propeptide that is activated by uPA or HGF Activator at sites of tissue damage. Its signaling through the receptor HGF R/c-MET is enhanced by its prior binding to heparan sulfate proteoglycans. The serum levels of HGF are elevated in a wide range of pathologies including liver damage, acute kidney failure, myocardial infarction, type 1 diabetes, obesity, and cancer, as well as in the synovial fluid of rheumatoid arthritis patients.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 150 µL of Assay Diluent to each well.
- Add 50 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 200 µL of Conjugate to each well.
- For Serum & Plasma Samples: Cover with a new plate sealer, and incubate at room temperature for 2 hours.
For Cell Culture Supernate Samples: Cover with a new plate sealer, and incubate at room temperature for 1.75 hours. - Aspirate and wash 4 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human HGF Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
92
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Rapid and effective preparation of clonal bone marrow-derived mesenchymal stem/stromal cell sheets to reduce renal fibrosis
Authors: S Kameishi, CM Dunn, M Oka, K Kim, YK Cho, SU Song, DW Grainger, T Okano
Scientific Reports, 2023-03-17;13(1):4421.
Species: Human
Sample Types: Cell Culture Supernates
-
Novel rAAV vector mediated intrathecal HGF delivery has an impact on neuroimmune modulation in the ALS motor cortex with TDP-43 pathology
Authors: B Genç, B Nho, H Seung, B Helmold, H Park, Ö Gözütok, S Kim, J Park, S Ye, H Lee, N Lee, SS Yu, S Kim, J Lee, H Özdinler
Gene Therapy, 2023-02-24;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Rilotumumab Resistance Acquired by Intracrine Hepatocyte Growth Factor Signaling
Authors: F Cecchi, K Rex, J Schmidt, CD Vocke, YH Lee, S Burkett, D Baker, MA Damore, A Coxon, TL Burgess, DP Bottaro
Cancers, 2023-01-11;15(2):.
Species: Xenograft
Sample Types: Serum
-
ARHGAP-RhoA signaling provokes homotypic adhesion-triggered cell death of metastasized diffuse-type gastric cancer
Authors: M Komatsu, H Ichikawa, F Chiwaki, H Sakamoto, R Komatsuzak, M Asaumi, K Tsunoyama, T Fukagawa, H Matsushita, N Boku, K Matsusaki, F Takeshita, T Yoshida, H Sasaki
Oncogene, 2022-09-20;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Mesenchymal Stem Cell Sheet Centrifuge-Assisted Layering Augments Pro-Regenerative Cytokine Production
Authors: S Bou-Ghanna, K Kim, M Kondo, DW Grainger, T Okano
Cells, 2022-09-12;11(18):.
Species: Human
Sample Types: Cell Culture Supernates
-
Hepatocyte growth factor derived from senescent cells attenuates cell competition-induced apical elimination of oncogenic cells
Authors: N Igarashi, K Miyata, TM Loo, M Chiba, A Hanyu, M Nishio, H Kawasaki, H Zheng, S Toyokuni, S Kon, K Moriyama, Y Fujita, A Takahashi
Oncogene, 2022-07-18;13(1):4157.
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine response over the course of COVID-19 infection in pregnant women
Authors: DB Rosen, EA Murphy, RS Gejman, A Capili, RL Friedlande, S Rand, KA Cagino, SM Glynn, KC Matthews, JM Kubiak, J Yee, M Prabhu, LE Riley, YJ Yang
Cytokine, 2022-04-25;154(0):155894.
Species: Human
Sample Types: Serum
-
Combination Therapy of Placenta-Derived Mesenchymal Stem Cells with WKYMVm Promotes Hepatic Function in a Rat Model with Hepatic Disease via Vascular Remodeling
Authors: JH Jun, S Park, JY Kim, JY Lim, GT Park, JH Kim, GJ Kim
Cells, 2022-01-11;11(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Freezing of cell sheets using a 3D freezer produces high cell viability after thawing
Authors: K Ueno, S Ike, N Yamamoto, Y Matsuno, H Kurazumi, R Suzuki, S Katsura, B Shirasawa, K Hamano
Biochemistry and Biophysics Reports, 2021-11-08;28(0):101169.
Species: Human
Sample Types: Cell culture supernate
-
HGF/MET in osteogenic differentiation of primary human palatal periosteum-derived mesenchymal stem cells
Authors: NY Naung, WJ Duncan, RK De Silva, DE Coates
Journal of oral science, 2021-09-15;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Adipose-derived mesenchymal stem cells preserve cardiac function via ANT-1 in dilated cardiomyopathy hamster model
Authors: D Mori, S Miyagawa, T Kido, H Hata, T Ueno, K Toda, T Kuratani, M Oota, K Kawai, H Kurata, H Nishida, Y Sawa
Regenerative Therapy, 2021-07-09;18(0):182-190.
Species: Human
Sample Types: Cell Culture Supernates
-
Defined MSC exosome with high yield and purity to improve regenerative activity
Authors: JY Kim, WK Rhim, YI Yoo, DS Kim, KW Ko, Y Heo, CG Park, DK Han
Journal of tissue engineering, 2021-04-20;12(0):2041731421100.
Species: Human
Sample Types: Cell Lysates
-
Suppression of Peritoneal Fibrosis by Sonoporation of Hepatocyte Growth Factor Gene-Encoding Plasmid DNA in Mice
Authors: K Nishimura, K Ogawa, M Kawaguchi, S Fumoto, H Mukai, S Kawakami
Pharmaceutics, 2021-01-18;13(1):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Crosstalk between Mast Cells and Lung Fibroblasts Is Modified by Alveolar Extracellular Matrix and Influences Epithelial Migration
Authors: M Bagher, O Rosmark, L Elowsson R, A Nybom, S Wasserstro, C Müller, XH Zhou, G Dellgren, O Hallgren, L Bjermer, AK Larsson-Ca, G Westergren
International Journal of Molecular Sciences, 2021-01-06;22(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Therapeutic Angiogenesis by a "Dynamic Duo": Simultaneous Expression of HGF and VEGF165 by Novel Bicistronic Plasmid Restores Blood Flow in Ischemic Skeletal Muscle
Authors: E Slobodkina, M Boldyreva, M Karagyaur, R Eremichev, N Alexandrus, V Balabanyan, Z Akopyan, Y Parfyonova, V Tkachuk, P Makarevich
Pharmaceutics, 2020-12-18;12(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Peritumoral activated hepatic stellate cells are associated with hepatic recurrence for resectable colorectal adenocarcinoma liver metastasis following resection
Authors: L Deng, T Li, Y Liao, S Liu, Z Xie, Z Huang, H Dai, J Li, X Lei
Oncol Lett, 2020-09-23;20(6):287.
Species: Human
Sample Types: Cell Culture Supernates
-
Autocrine HGF/c-Met signaling pathway confers aggressiveness in lymph node adult T-cell leukemia/lymphoma
Authors: H Totani, K Shinjo, M Suzuki, K Katsushima, S Mase, A Masaki, A Ito, M Ri, S Kusumoto, H Komatsu, T Ishida, H Inagaki, S Iida, Y Kondo
Oncogene, 2020-08-04;0(0):.
Species: Human
Sample Types: Cell Culture Supernates, Serum
-
Repeated Freezing Procedures Preserve Structural and Functional Properties of Amniotic Membrane for Application in Ophthalmology
Authors: O Pogozhykh, N Hofmann, O Gryshkov, C von Kaisen, M Mueller, B Glasmacher, D Pogozhykh, M Börgel, R Blasczyk, C Figueiredo
Int J Mol Sci, 2020-06-04;21(11):.
Species: Human
Sample Types: Tissue Homogenates
-
Detection of hepatocyte growth factor in oral rinses using water for possible periodontal diagnosis
Authors: S Suzuki, A Aoki, S Katagiri, S Maekawa, K Ejiri, S Kong, M Nagata, Y Yamaguchi, M Ohshima, Y Izumi
J Oral Sci, 2020-05-15;0(0):.
Species: Human
Sample Types: Oral Rinse
-
Aberrant activation of hepatocyte growth factor/MET signaling promotes beta-catenin-mediated prostatic tumorigenesis
Authors: J Aldahl, J Mi, A Pineda, WK Kim, A Olson, E Hooker, Y He, EJ Yu, V Le, DH Lee, J Geradts, Z Sun
J. Biol. Chem., 2019-12-09;295(2):631-644.
Species: Mouse
Sample Types: Serum
-
Hypoxic Preconditioning Enhances Survival and Proangiogenic Capacity of Human First Trimester Chorionic Villus-Derived Mesenchymal Stem Cells for Fetal Tissue Engineering
Authors: D Hao, C He, B Ma, L Lankford, L Reynaga, DL Farmer, F Guo, A Wang
Stem Cells Int, 2019-11-12;2019(0):9695239.
Species: Human
Sample Types: Cell Culture Supernates
-
Human mesenchymal stem cell sheets in xeno-free media for possible allogenic applications
Authors: K Kim, S Bou-Ghanna, H Thorp, DW Grainger, T Okano
Sci Rep, 2019-10-08;9(1):14415.
Species: Human
Sample Types: Cell Culture Supernates
-
Bioinspired Self-assembling Peptide Hydrogel with Proteoglycan-assisted Growth Factor Delivery for Therapeutic Angiogenesis
Authors: LC Huang, HC Wang, LH Chen, CY Ho, PH Hsieh, MY Huang, HC Wu, TW Wang
Theranostics, 2019-09-21;9(23):7072-7087.
Species: Human
Sample Types: Hydrogel Supernates
-
Extracellular lipid loading augments hypoxic paracrine signaling and promotes glioma angiogenesis and macrophage infiltration
Authors: S Offer, JA Menard, JE Pérez, KG de Oliveir, VI Chandran, MC Johansson, A Bång-Ruden, P Siesjö, A Ebbesson, I Hedenfalk, PC Sundgren, A Darabi, M Belting
J. Exp. Clin. Cancer Res., 2019-06-07;38(1):241.
Species: Human
Sample Types: Cell Culture Supernates
-
Characteristic differences of cell sheets composed of mesenchymal stem cells with different tissue origins
Authors: M Nakao, D Inanaga, K Nagase, H Kanazawa
Regen Ther, 2019-05-10;11(0):34-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased hepatocyte growth factor levels over 2 years are associated with coronary heart disease to Increased hepatocyte growth factor levels over 2 years are associated with coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA)
Authors: PA Decker, NB Larson, EJ Bell, JS Pankow, NQ Hanson, CL Wassel, MY Tsai, SJ Bielinski
Am. Heart J., 2019-04-13;213(0):30-34.
Species: Human
Sample Types: Serum
-
Human serum and platelet lysate are appropriate xeno-free alternatives for clinical-grade production of human MuStem cell batches
Authors: C Saury, A Lardenois, C Schleder, I Leroux, B Lieubeau, L David, M Charrier, L Guével, S Viau, B Delorme, K Rouger
Stem Cell Res Ther, 2018-05-02;9(1):128.
Species: Human
Sample Types: Cell Culture Supernates
-
Forty-nine gastric cancer cell lines with integrative genomic profiling for development of c-MET inhibitor
Authors: HJ Kim, SK Kang, WS Kwon, TS Kim, I Jeong, HC Jeung, M Kragh, ID Horak, HC Chung, SY Rha
Int. J. Cancer, 2018-02-23;0(0):.
Species: Human
Sample Types: Cell Lysates
-
Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-?B signaling pathway in vivo and in vitro
Authors: B Liu, F Ding, D Hu, Y Zhou, C Long, L Shen, Y Zhang, D Zhang, G Wei
Stem Cell Res Ther, 2018-01-12;9(1):7.
Species: Human
Sample Types: Cell Culture Supernates
-
miRNA profiling of NurOwn�: mesenchymal stem cells secreting neurotrophic factors
Authors: Y Gothelf, H Kaspi, N Abramov, R Aricha
Stem Cell Res Ther, 2017-11-07;8(1):249.
Species: Human
Sample Types: Cell Culture Supernates
-
Exogenous growth factors bFGF, EGF and HGF do not influence viability and phenotype of V600EBRAF melanoma cells and their response to vemurafenib and trametinib in vitro
Authors: I Zalesna, M Osrodek, ML Hartman, M Rozanski, M Sztiller-S, K Niewinna, D Nejc, M Czyz
PLoS ONE, 2017-08-22;12(8):e0183498.
Species: Human
Sample Types: Whole Cells
-
Human hepatocyte growth factor inhibits early neointima formation in rabbit abdominal aortae following ultrasound-guided balloon injury
Authors: L Mei, Y He, H Wang, Y Jin, S Wang, C Jin
Mol Med Rep, 2017-08-11;0(0):.
Species: Rabbit
Sample Types: Tissue Homogenates
-
Five-weekly S-1 plus cisplatin therapy combined with trastuzumab therapy in HER2-positive gastric cancer: a phase II trial and biomarker study (WJOG7212G)
Authors: Y Miura, Y Sukawa, S Hironaka, M Mori, K Nishikawa, S Tokunaga, H Okuda, T Sakamoto, K Taku, K Nishikawa, T Moriwaki, Y Negoro, Y Kimura, K Uchino, K Shinozaki, H Shinozaki, N Musha, H Yoshiyama, T Tsuda, Y Miyata, N Sugimoto, T Shirakawa, M Ito, K Yonesaka, K Yoshimura, N Boku, K Nosho, T Takano, I Hyodo
Gastric Cancer, 2017-05-11;0(0):.
Species: Human
Sample Types: Serum
-
Comparison of fibrin glue and Vicryl sutures in conjunctival autografting for pterygium surgery
Authors: X Wang, Y Zhang, L Zhou, R Wei, L Dong
Mol. Vis., 2017-04-19;23(0):275-285.
Species: Human
Sample Types: Tears
-
Preclinical development of a humanized neutralizing antibody targeting HGF
Authors: H Kim, SH Hong, JY Kim, IC Kim, YW Park, SJ Lee, SW Song, JJ Kim, G Park, TM Kim, YH Kim, JB Park, J Chung, IH Kim
Exp. Mol. Med, 2017-03-24;49(3):e309.
Species: Human
Sample Types: Serum
-
Hypoxic preconditioning of human cardiosphere-derived cell sheets enhances cellular functions via activation of the PI3K/Akt/mTOR/HIF-1? pathway
Authors: Y Tanaka, T Hosoyama, A Mikamo, H Kurazumi, A Nishimoto, K Ueno, B Shirasawa, K Hamano
Am J Transl Res, 2017-02-15;9(2):664-673.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of Hyperglycemia and Low Oxygen Tension on Adipose-Derived Stem Cells Compared with Dermal Fibroblasts and Keratinocytes: Importance for Wound Healing in Type 2 Diabetes
PLoS ONE, 2016-12-19;11(12):e0168058.
Species: Human
Sample Types: Cell Culture Supernates
-
Erythropoietin induces production of hepatocyte growth factor from bone marrow mesenchymal stem cells in�vitro
Authors: Majid Mossahebi-
Biologicals, 2016-11-16;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Adipose-derived mesenchymal stem cells from patients with atherosclerotic renovascular disease have increased DNA damage and reduced angiogenesis that can be modified by hypoxia
Stem Cell Res Ther, 2016-09-09;7(1):128.
Species: Human
Sample Types: Cell Culture Supernates
-
Neovascularization Potential of Blood Outgrowth Endothelial Cells From Patients With Stable Ischemic Heart Failure Is Preserved
Authors: D Dauwe, B Pelacho, A Wibowo, AS Walravens, K Verdonck, H Gillijns, E Caluwe, P Pokreisz, N van Gastel, G Carmeliet, M Depypere, F Maes, N Vanden Dri, W Droogne, J Van Cleemp, J Vanhaecke, F Prosper, C Verfaillie, A Luttun, S Janssens
J Am Heart Assoc, 2016-04-18;5(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
M-CSF in a new biomarker panel with HE4 and CA 125 in the diagnostics of epithelial ovarian cancer patients.
Authors: Bedkowska G, Lawicki S, Gacuta E, Pawlowski P, Szmitkowski M
J Ovarian Res, 2015-05-03;8(0):27.
Species: Human
Sample Types: Plasma
-
The role of HGF/MET and FGF/FGFR in fibroblast-derived growth stimulation and lapatinib-resistance of esophageal squamous cell carcinoma.
Authors: Saito S, Morishima K, Ui T, Hoshino H, Matsubara D, Ishikawa S, Aburatani H, Fukayama M, Hosoya Y, Sata N, Lefor A, Yasuda Y, Niki T
BMC Cancer, 2015-02-25;15(0):82.
Species: Human
Sample Types: Cell Culture Supernates
-
Multiple regulatory mechanisms of hepatocyte growth factor expression in malignant cells with a short poly(dA) sequence in the HGF gene promoter.
Authors: Sakai K, Takeda M, Okamoto I, Nakagawa K, Nishio K
Oncol Lett, 2014-11-11;9(1):405-410.
Species: Human
Sample Types: Cell Culture Supernates
-
Diversity of biological effects induced by longwave UVA rays (UVA1) in reconstructed skin.
Authors: Marionnet C, Pierrard C, Golebiewski C, Bernerd F
PLoS ONE, 2014-08-20;9(8):e105263.
Species: Human
Sample Types: Cell Culture Supernates
-
Combined targeting of mTOR and c-MET signaling pathways for effective management of epithelioid sarcoma.
Authors: Imura, Yoshinor, Yasui, Hirohiko, Outani, Hidetats, Wakamatsu, Toru, Hamada, Kenichir, Nakai, Takaaki, Yamada, Shutaro, Myoui, Akira, Araki, Nobuhito, Ueda, Takafumi, Itoh, Kazuyuki, Yoshikawa, Hideki, Naka, Norifumi
Mol Cancer, 2014-08-07;13(0):185.
Species: Human, Mouse
Sample Types: Cell Culture Supernates, Serum
-
Ex vivo expansion of bovine corneal endothelial cells in xeno-free medium supplemented with platelet releasate.
Authors: Chou M, Burnouf T, Wang T
PLoS ONE, 2014-06-19;9(6):e99145.
Species: Human
Sample Types: Cell Culture Supernates
-
Establishment of a novel clear cell sarcoma cell line (Hewga-CCS), and investigation of the antitumor effects of pazopanib on Hewga-CCS.
Authors: Outani, Hidetats, Tanaka, Takaaki, Wakamatsu, Toru, Imura, Yoshinor, Hamada, Kenichir, Araki, Nobuhito, Itoh, Kazuyuki, Yoshikawa, Hideki, Naka, Norifumi
BMC Cancer, 2014-06-19;14(0):455.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum levels of hepatocyte growth factor and epiregulin are associated with the prognosis on anti-EGFR antibody treatment in KRAS wild-type metastatic colorectal cancer.
Authors: Takahashi, N, Yamada, Y, Furuta, K, Honma, Y, Iwasa, S, Takashima, A, Kato, K, Hamaguchi, T, Shimada, Y
Br J Cancer, 2014-05-06;110(11):2716-27.
Species: Human
Sample Types: Serum
-
Effects of high intensity training and high volume training on endothelial microparticles and angiogenic growth factors.
Authors: Wahl P, Jansen F, Achtzehn S, Schmitz T, Bloch W, Mester J, Werner N
PLoS ONE, 2014-04-25;9(4):e96024.
Species: Human
Sample Types: Serum
-
The molecular mechanism underlying the proliferating and preconditioning effect of vitamin C on adipose-derived stem cells.
Authors: Kim, Ji Hye, Kim, Wang-Kyu, Sung, Young Kw, Kwack, Mi Hee, Song, Seung Yo, Choi, Joon-Seo, Park, Sang Gyu, Yi, TacGhee, Lee, Hyun-Joo, Kim, Dae-Duk, Seo, Hyun Min, Song, Sun U, Sung, Jong-Hyu
Stem Cells Dev, 2014-03-21;23(12):1364-76.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro hepatic trans-differentiation of human mesenchymal stem cells using sera from congestive/ischemic liver during cardiac failure.
Authors: Bishi, Dillip K, Mathapati, Santosh, Cherian, Kotturat, Guhathakurta, Soma, Verma, Rama Sha
PLoS ONE, 2014-03-18;9(3):e92397.
Species: Human
Sample Types: Serum
-
A pharmacodynamic/pharmacokinetic study of ficlatuzumab in patients with advanced solid tumors and liver metastases.
Authors: Tabernero J, Elez M, Herranz M, Rico I, Prudkin L, Andreu J, Mateos J, Carreras M, Han M, Gifford J, Credi M, Yin W, Agarwal S, Komarnitsky P, Baselga J
Clin Cancer Res, 2014-03-14;20(10):2793-804.
Species: Human
Sample Types: Serum
-
Gene expression profiling and secretome analysis differentiate adult-derived human liver stem/progenitor cells and human hepatic stellate cells.
Authors: Berardis S, Lombard C, Evraerts J, El Taghdouini A, Rosseels V, Sancho-Bru P, Lozano J, van Grunsven L, Sokal E, Najimi M
PLoS ONE, 2014-01-21;9(1):e86137.
Species: Human
Sample Types: Cell Culture Supernates
-
Analysis of plasma cytokines and angiogenic factors in patients with pretreated urothelial cancer receiving Pazopanib: the role of circulating interleukin-8 to enhance the prognostic accuracy.
Authors: Necchi A, Pennati M, Zaffaroni N, Landoni E, Giannatempo P, Raggi D, Schwartz L, Morosi C, Crippa F, Fare E, Nicolai N, Lanocita R, Sava T, Sacco C, Messina C, Ortega C, De Braud F, Salvioni R, Daidone M, Gianni A, Mariani L
Br J Cancer, 2013-11-14;110(1):26-33.
Species: Human
Sample Types: Plasma
-
Biological, functional and genetic characterization of bone marrow-derived mesenchymal stromal cells from pediatric patients affected by acute lymphoblastic leukemia.
Authors: Conforti A, Biagini S, Del Bufalo F, Sirleto P, Angioni A, Starc N, Li Pira G, Moretta F, Proia A, Contoli B, Genovese S, Ciardi C, Avanzini M, Rosti V, Lo-Coco F, Locatelli F, Bernardo M
PLoS ONE, 2013-11-07;8(11):e76989.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro.
Authors: Li Q, Zhang A, Tao C, Li X, Jin P
Biochem Biophys Res Commun, 2013-10-30;441(3):675-80.
Species: Human
Sample Types: Cell Culture Supernates
-
Functional and differential proteomic analyses to identify platelet derived factors affecting ex vivo expansion of mesenchymal stromal cells.
Authors: Kinzebach S, Dietz L, Kluter H, Thierse H, Bieback K
BMC Cell Biol, 2013-10-30;14(0):48.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical outcome, proteome kinetics and angiogenic factors in serum after thermoablation of colorectal liver metastases.
Authors: Wertenbroek, Marieke, Schepers, Marianne, Kamminga-Rasker, Hannetta, Bottema, Jan T, Muller Kobold, Anneke C, Roelofsen, Han, de Jong, Koert P
BMC Cancer, 2013-05-30;13(1):266.
Species: Human
Sample Types: Serum
-
Hepatocyte growth factor pathway upregulation in the bone marrow microenvironment in multiple myeloma is associated with lytic bone disease.
Authors: Kristensen I, Christensen J, Lyng M, Moller M, Pedersen L, Rasmussen L, Ditzel H, Abildgaard N
Br J Haematol, 2013-02-22;161(3):373-82.
Species: Human
Sample Types: Plasma
-
Measuring urinary tubular biomarkers in type 2 diabetes does not add prognostic value beyond established risk factors.
Kidney Int, 2012-06-20;82(7):812-8.
Species: Human
Sample Types: Serum
-
Comparison of the levels of the growth factors in umbilical cord serum and human milk and its clinical significance.
Authors: Patki S, Patki U, Patil R, Indumathi S, Kaingade P, Bulbule A, Nikam A, Pishte A
Cytokine, 2012-05-19;59(2):305-8.
Species: Human
Sample Types: Serum
-
Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma.
Authors: Llovet JM, Pena CE, Lathia CD, Shan M, Meinhardt G, Bruix J, Pallota MG, Zarba JJ, Boyer M, Riordan S, Strickland A, Tebbutt N, Thomson B, Borbath I, De Greve J, Van Laethem J-, Van Steenbergen W, Van Vlierberghe H, Barrios C, Cosme de Oliveira A, Kotzev I, Takov D, Tchernev K, Burak K, Ma M, Metrakos P, Olweny C, Sherman M, Gamargo Garate C, Martinez-Castillo J, Beaugrand M, Bennouna J, Blanc J-, Bronowicki J-, Degos F, Dominguez S, Grange J-, Hillon P, Raoul J-, Seitz J-, Blum H, Buggisch P, Caspary W, Dollinger M, Gerken G, Goke B, Gregor M, Greten T, Haussinger D, Hilgard P, Scherübl J, Scheulen M, Schmid R, Spengler U, Wiest R, Zeuzem S, Arvanitakis C, Germanidis G, Katsos I, Figer A, Stemmer S, Amadori D, Bolondi L, Cognetti F, Craxi A, Farinati F, Gridelli C, Martoni A, Mazzaferro V, Porta C, Ricci S, Sangiovanni A, Santoro A, Trevisani F, Cisnero Garza LE, Gane E, O'Donnell A, Leon J, Lozano A, Jassem J, Rydzewska G, Szawlowski A, Tomczak P, Badulescu F, Miron L, Kubyshkin V, Bruix J, Forner A, Bustamante Schneider J, Diago M, Montero Alvarez JL, Pascual S, RuÃÂz del Arbol L, Sangro B, Solá R, Tabernero J, Muellhaupt B, Roth A, Jeffry Evans TR, Falk S, Meyer T, Reeves H, Ross P, Befeler A, Boyer T, Britten C, Byrne T, Garcia-Tsao G, Gold P, Goldenberg A, Heuman D, Kennedy P, Koch A, Llovet JM, Marrero J, Schilsky M, Schwartz J, Schwartz M
Clin. Cancer Res., 2012-02-28;18(8):2290-300.
Species: Human
Sample Types: Plasma
-
The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells.
Authors: Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T, Kuper M, Stock UA, Staiger H, Machicao F, Schaller HE, Konigsrainer A, Haring HU, Siegel-Axel DI
Diabetologia, 2012-02-13;55(5):1514-25.
Species: Human
Sample Types: Serum
-
Validation and quality control of ELISAs for the use with human saliva samples.
Authors: Jaedicke KM, Taylor JJ, Preshaw PM
J. Immunol. Methods, 2012-01-28;377(1):62-5.
Species: Human
Sample Types: Saliva
-
Hypoxia-inducible factors in human pulmonary arterial hypertension: a link to the intrinsic myeloid abnormalities.
Authors: Farha S, Asosingh K, Xu W, Sharp J, George D, Comhair S, Park M, Tang WH, Loyd JE, Theil K, Tubbs R, Hsi E, Lichtin A, Erzurum SC
Blood, 2011-01-21;117(13):3485-93.
Species: Human
Sample Types: Plasma
-
Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media.
Authors: Rauch C, Feifel E, Amann EM, Spotl HP, Schennach H, Pfaller W, Gstraunthaler G
ALTEX, 2011-01-01;28(4):305-16.
Species: Human
Sample Types: Cell Lysates
-
Human stem/progenitor cells from bone marrow enhance glial differentiation of rat neural stem cells: a role for transforming growth factor beta and Notch signaling.
Authors: Robinson AP, Foraker JE, Ylostalo J, Prockop DJ
Stem Cells Dev., 2010-09-14;20(2):289-300.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of serum hepatocyte growth factor on treatment response to epidermal growth factor receptor tyrosine kinase inhibitors in patients with non-small cell lung adenocarcinoma.
Authors: Kasahara K, Arao T, Sakai K, Matsumoto K, Sakai A, Kimura H, Sone T, Horiike A, Nishio M, Ohira T, Ikeda N, Yamanaka T, Saijo N, Nishio K
Clin. Cancer Res., 2010-08-02;16(18):4616-24.
Species: Human
Sample Types: Serum
-
Expansion of human cardiac stem cells in physiological oxygen improves cell production efficiency and potency for myocardial repair.
Authors: Li TS, Cheng K, Malliaras K, Matsushita N, Sun B, Marban L, Zhang Y, Marban E
Cardiovasc. Res., 2010-07-29;89(1):157-65.
Species: Human
Sample Types: Cell Culture Supernates
-
Wnt activity defines colon cancer stem cells and is regulated by the microenvironment.
Authors: Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP
Nat. Cell Biol., 2010-04-25;12(5):468-76.
Species: Human
Sample Types: Cell Culture Supernates
-
Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors.
Authors: Gordon MS, Sweeney CS, Mendelson DS
Clin. Cancer Res., 2010-01-12;16(2):699-710.
Species: Human
Sample Types: Plasma
-
Alternative proteolytic processing of hepatocyte growth factor during wound repair.
Authors: Buchstein N, Hoffmann D, Smola H, Lang S, Paulsson M, Niemann C, Krieg T, Eming SA
Am. J. Pathol., 2009-04-23;174(6):2116-28.
Species: Human
Sample Types: Tissue Secretion
-
IFATS Series: FGF-2-induced HGF Secretion By Adipose-Derived Stromal Cells Inhibits Post-Injury Fibrogenesis Through A JNK-Dependent Mechanism.
Authors: Suga H, Eto H, Shigeura T, Inoue K, Aoi N, Kato H, Nishimura S, Manabe I, Gonda K, Yoshimura K
Stem Cells, 2009-01-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Abnormal cytokine production by bone marrow stromal cells of multiple myeloma patients in response to RPMI8226 myeloma cells.
Authors: Zdzisinska B, Bojarska-Junak A, Dmoszynska A, Kandefer-Szerszen M
Arch. Immunol. Ther. Exp. (Warsz.), 2008-05-30;56(3):207-21.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical correlations and prognostic relevance of HGF, VEGF AND FGF expression in Brazilian patients with non-Hodgkin lymphoma.
Authors: Etto L, Lacerda E, Baiocchi O, Silva V, Dalboni M, Alves A, Silva M, Vettore A, Colleoni G
Leuk. Lymphoma, 2008-02-01;49(2):257-64.
Species: Human
Sample Types: Serum
-
Impairment of the antifibrotic effect of hepatocyte growth factor in lung fibroblasts from African Americans: possible role in systemic sclerosis.
Authors: Bogatkevich GS, Ludwicka-Bradley A, Highland KB, Hant F, Nietert PJ, Singleton CB, Feghali-Bostwick CA, Silver RM
Arthritis Rheum., 2007-07-01;56(7):2432-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Expression of Tie-2 and other receptors for endothelial growth factors in acute myeloid leukemias is associated with monocytic features of leukemic blasts.
Authors: Riccioni R, Diverio D, Mariani G, Buffolino S, Riti V, Saulle E, Petrucci E, Cedrone M, Lo-Coco F, Foa R, Peschle C, Testa U
Stem Cells, 2007-04-19;25(8):1862-71.
Species: Human
Sample Types: Cell Culture Supernates
-
Hepatocyte growth factor levels in gingival crevicular fluid in health, disease, and after treatment.
Authors: Nagaraja C, Pradeep AR
J. Periodontol., 2007-04-01;78(4):742-7.
Species: Human
Sample Types:
-
A fusion protein of hepatocyte growth factor for immobilization to collagen.
Authors: Kitajima T, Terai H, Ito Y
Biomaterials, 2007-01-19;28(11):1989-97.
Species: Human
Sample Types: Recombinant Protein
-
Platelet-derived growth factor-BB controls epithelial tumor phenotype by differential growth factor regulation in stromal cells.
Authors: Lederle W, Stark HJ, Skobe M, Fusenig NE, Mueller MM
Am. J. Pathol., 2006-11-01;169(5):1767-83.
Species: Human
Sample Types: Cell Culture Supernates
-
Humoral and cellular factors responsible for coronary collateral formation.
Authors: Sherman JA, Hall A, Malenka DJ, De Muinck ED, Simons M
Am. J. Cardiol., 2006-08-31;98(9):1194-7.
Species: Human
Sample Types: Plasma
-
Relation of various plasma growth factor levels in patients with stable angina pectoris and total occlusion of a coronary artery to the degree of coronary collaterals.
Authors: Briguori C, Testa U, Colombo A, Petrucci E, Condorelli G, Airoldi F, Peschle C, Condorelli G
Am. J. Cardiol., 2005-12-20;97(4):472-6.
Species: Human
Sample Types: Serum
-
IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis.
Authors: Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H
J. Immunol., 2005-11-01;175(9):6177-89.
Species: Human
Sample Types: Cell Culture Supernates
-
Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells.
Authors: Rehman J, Li J, Parvathaneni L, Karlsson G, Panchal VR, Temm CJ, Mahenthiran J, March KL
J. Am. Coll. Cardiol., 2004-06-16;43(12):2314-8.
Species: Human
Sample Types: Plasma
-
Differential macrophage infiltration in early and advanced endometriosis and adjacent peritoneum.
Authors: Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T
Fertil. Steril., 2004-03-01;81(3):652-61.
Species: Human
Sample Types: Cell Culture Supernates
-
Hepatocyte growth factor in polymorphonuclear leukocytes is increased in patients with systemic inflammatory response syndrome.
Authors: Matsushima A, Ogura H, Koh T, Fujita K, Yoshiya K, Sumi Y, Hosotsubo H, Kuwagata Y, Tanaka H, Shimazu T, Sugimoto H
J Trauma, 2004-02-01;56(2):259-64.
Species: Human
Sample Types: Plasma
-
Hepatocyte growth factor and its role in the pathogenesis of retinal detachment.
Authors: Jin M, Chen Y, He S, Ryan SJ, Hinton DR
Invest. Ophthalmol. Vis. Sci., 2004-01-01;45(1):323-9.
Species: Rabbit
Sample Types: Vitreous Humor
-
Hepatocyte growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy: its relationship with vascular endothelial growth factor and retinopathy activity.
Authors: Simo R, Lecube A, Garcia-Arumi J, Carrasco E, Hernandez C
Diabetes Care, 2004-01-01;27(1):287-8.
Species: Human
Sample Types: Vitreous Humor
-
Hepatocyte growth factor (HGF) system in gingiva: HGF activator expression by gingival epithelial cells.
Authors: Ohshima M, ù °, Sakai A, Sawamoto Y, Seki K, Ito K, Otsuka K
2002-12-01;44(3):129-34.
Species: Human
Sample Types:
-
Association of interleukin-6 and estradiol with hepatocyte growth factor in peritoneal fluid of women with endometriosis.
Authors: Khan KN, Masuzaki H, Fujishita A, Hamasaki T, Kitajima M, Hasuo A, Miyamura Y, Ishimaru T
Acta Obstet Gynecol Scand, 2002-08-01;81(8):764-71.
Species: Human
Sample Types: Peritoneal Fluid
-
Selective plasma filtration for treatment of fulminant hepatic failure induced by D-galactosamine in a pig model.
Authors: Ho DW, Fan ST, To J, Woo YH, Zhang Z, Lau C, Wong J
Gut, 2002-06-01;50(6):869-76.
Species: Porcine
Sample Types: Plasma
-
Exhaled breath condensate and serum levels of hepatocyte growth factor in pneumonia.
Authors: Nayeri F, Millinger E, Nilsson I, Zetterström, Brudin L, Forsberg P
Respir Med, 2002-02-01;96(2):115-9.
Species: Human
Sample Types: Exhaled Breath Condensate (EBC)
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human HGF Quantikine ELISA Kit
Average Rating: 4.5 (Based on 2 Reviews)
Have you used Human HGF Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: