Human IFN-gamma DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
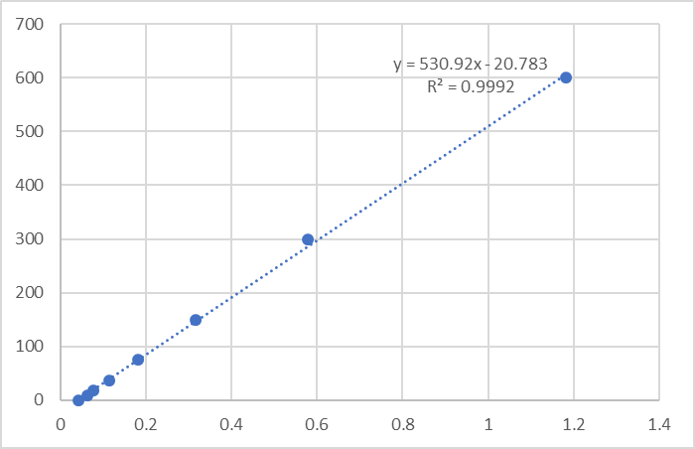
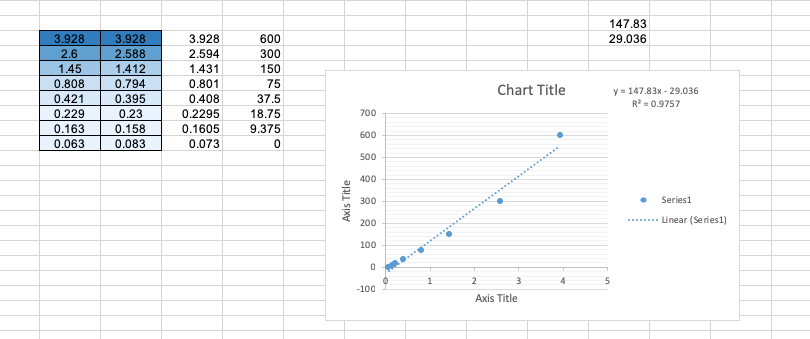
Scientific Data
Preparation and Storage
Background: IFN-gamma
IFN-gamma (Interferon-gamma) is the prototype proinflammatory cytokine and is produced by a variety of immune cells under inflammatory conditions, notably by T cells and NK cells. It plays a key role in host defense by promoting the development and activation of Th1 cells, chemoattraction and activation of monocytes and macrophages, upregulation of antigen presentation molecules, and immunoglobulin class switching in B cells. It also exhibits antiviral, antiproliferative, and apoptotic effects. In addition, IFN-gamma functions as an anti-inflammatory mediator by promoting the development of regulatory T cells and inhibiting Th17 cell differentiation. IFN-gamma dimers signal through a receptor complex of two IFN-gamma R1 and two IFN-gamma R2 subunits.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL of Block Buffer to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human IFN-gamma DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
226
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Recurrent immunogenic neoantigens and their cognate T-cell receptors in treatment-resistant metastatic prostate cancer
Authors: Gumpert, N;Sagie, S;Arnedo-Pac, C;Babu, T;Weller, C;Gonzalez-Perez, A;Wang, Y;Michel Todó, L;Levy, R;Chen, X;Greenberg, P;Dayan-Rubinov, M;Yakubovich, E;Wasserman-Bartov, T;Zerbib, M;Gong, J;Rebernick, RJ;Oliveira Tercero, A;Agundez Muriel, L;Benedek, G;Kedmi, M;Oren, R;Ben-Dor, S;Levin, Y;Troyanskaya, OG;Munzur, AD;Wyatt, AW;Cieslik, MP;Quigley, DA;Van Allen, EM;Anandasabapathy, N;Mateo, J;Yang, X;Martínez-Jiménez, F;Lopez-Bigas, N;Samuels, Y;
Cancer discovery
Species: Human
Sample Types: Cell Culture Supernates
-
DNA 5-Hydroxymethylcytosine Landscape and Transcriptional Profile Highlight the TUBB4B-Mediated Th17/Th1/Treg Imbalance in Behçet's Uveitis
Authors: Zhang, W;Zhang, P;Pu, Y;Chen, Z;Su, G;Deng, Y;Zhang, Y;Ji, Y;Huang, Z;Zhou, Q;Luo, X;Lai, Y;Yang, P;
Investigative ophthalmology & visual science
Species: Human
Sample Types: Cell Culture Supernates
-
IFN?-inducible Gbp4 and Irgb6 contribute to experimental cerebral malaria pathology in the olfactory bulb
Authors: Matsuo-Dapaah, J;Alshaweesh, J;Lee, MSJ;Hayashi, T;Dash, R;Kuroda, M;Tainaka, K;Ozawa, M;Kuratani, A;Yamamoto, M;Liu, K;Fukui, R;Miyake, K;Kobiyama, K;Rénia, L;Ishii, KJ;Coban, C;
mBio
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Ex Vivo Overactivation of Lymphocyte Subsets in Fibrotic Hypersensitivity Pneumonitis Is Blunted by a Sphingosine-1-Phosphate Receptor Ligand
Authors: Courtemanche, O;Huppé, CA;Blais-Lecours, P;Maranda, C;Morissette, MC;Blanchet, MR;Dion, G;Marsolais, D;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Plasma Proteomic Signature as a Predictor of Age Advancement in People Living With HIV
Authors: Navas, A;Matzaraki, V;van Eekeren, LE;Blaauw, MJT;Groenendijk, AL;Vos, WAJW;Jacobs-Cleophas, M;Dos Santos, JC;van der Ven, AJAM;Joosten, LAB;Netea, MG;
Aging cell
Species: Human
Sample Types: Cell Culture Supernates
-
A Novel Approach for In Vitro Testing and Hazard Evaluation of Nanoformulated RyR2-Targeting siRNA Drugs Using Human PBMCs
Authors: Bettinsoli, V;Melzi, G;Crea, A;Esposti, L;Iafisco, M;Catalucci, D;Ciana, P;Corsini, E;
Life
Species: Human
Sample Types: Cell Culture Supernates
-
Trabectedin enhances the antitumor effects of IL-12 in triple-negative breast cancer
Authors: Schwarz, E;Savardekar, H;Zelinskas, S;Mouse, A;Lapurga, G;Lyberger, J;Rivaldi, A;Ringwalt, EM;Miller, KE;Yu, L;Behbehani, GK;Cripe, TP;Carson, WE;
Cancer immunology research
Species: Human
Sample Types: Cell Culture Supernates
-
NI-3201 Is a Bispecific Antibody Mediating PD-L1-Dependent CD28 Co-stimulation on T Cells for Enhanced Tumor Control
Authors: Majocchi, S;Lloveras, P;Nouveau, L;Legrand, M;Viandier, A;Malinge, P;Charreton, M;Raymond, C;Pace, E;Millard, B;Svensson, L;Kelpas, V;Anceriz, N;Salgado-Pires, S;Daubeuf, B;Magistrelli, G;Gueneau, F;Moine, V;Masternak, K;Shang, L;Fischer, N;Ferlin, W;
Cancer Immunology Research
Species: Human
Sample Types: Cell Culture Supernates
-
Exploring the innate immune response in polycystic liver disease
Authors: Duijzer, R;Dalloyaux, D;Boerrigter, MM;Lemmers, H;Dijkstra, H;van Emst, L;Te Morsche, RHM;Jaeger, M;Joosten, LAB;Drenth, JPH;
Cytokine
Species: Human
Sample Types: Cell Culture Supernates
-
Paired CRISPR screens to map gene regulation in cis and trans
Authors: Xue, X;Gajic, ZZ;Caragine, CM;Legut, M;Walker, C;Kim, JYS;Wang, X;Yan, RE;Wessels, HH;Lu, C;Bapodra, N;Gürsoy, G;Sanjana, NE;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Characteristics of Pulmonary Inflammation in Patients with Different Forms of Active Tuberculosis
Authors: Shepelkova, GS;Evstifeev, VV;Berezovskiy, YS;Ergeshova, AE;Tarasov, RV;Bagirov, MA;Yeremeev, VV;
International journal of molecular sciences
Species: Human
Sample Types: Tissue Homogenates
-
Proteasome inhibition enhances the anti-leukemic efficacy of chimeric antigen receptor (CAR) expressing NK cells against acute myeloid leukemia
Authors: Sedloev, D;Chen, Q;Unglaub, JM;Schanda, N;Hao, Y;Besiridou, E;Neuber, B;Schmitt, A;Raffel, S;Liu, Y;Janssen, M;Müller-Tidow, C;Schmitt, M;Sauer, T;
Journal of hematology & oncology
Species: Human
Sample Types: Cell Culture Supernates
-
The TGF? type I receptor kinase inhibitor vactosertib in combination with pomalidomide in relapsed/refractory multiple myeloma: a phase 1b trial
Authors: Malek, E;Rana, PS;Swamydas, M;Daunov, M;Miyagi, M;Murphy, E;Ignatz-Hoover, JJ;Metheny, L;Kim, SJ;Driscoll, JJ;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Trained immunity is regulated by T cell-induced CD40-TRAF6 signaling
Authors: Jacobs, MME;Maas, RJF;Jonkman, I;Negishi, Y;Tielemans Zamora, W;Yanginlar, C;van Heck, J;Matzaraki, V;Martens, JHA;Baltissen, M;Vermeulen, M;Morla-Folch, J;Ranzenigo, A;Wang, W;Umali, M;Ochando, J;van der Vlag, J;Hilbrands, LB;Joosten, LAB;Netea, MG;Mulder, WJM;van Leent, MMT;Mhlanga, MM;Teunissen, AJP;Rother, N;Duivenvoorden, R;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
CAR T-cell-mediated delivery of bispecific innate immune cell engagers for neuroblastoma
Authors: Pascual-Pasto, G;McIntyre, B;Hines, MG;Giudice, AM;Garcia-Gerique, L;Hoffmann, J;Mishra, P;Matlaga, S;Lombardi, S;Shraim, R;Schürch, PM;Yarmarkovich, M;Hofmann, TJ;Alikarami, F;Martinez, D;Tsang, M;Gil-de-Gómez, L;Spear, TT;Bernt, KM;Wolpaw, AJ;Dimitrov, DS;Li, W;Bosse, KR;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Dual A2A/ A2B adenosine receptor antagonist M1069 counteracts immuno-suppressive mechanisms of adenosine and reduces tumor growth in vivo
Authors: Schiemann, K;Belousova, N;Matevossian, A;Nallaparaju, KC;Kradjian, G;Pandya, M;Chen, Z;Aral, E;Krauel, EM;Petrova, E;Boesler, C;Kitzing, T;Lecomte, M;Wagner, C;Blayo, AL;Schann, S;Huck, B;Moisan, J;Zaynagetdinov, R;
Molecular cancer therapeutics
Species: Human
Sample Types: Cell Culture Supernates
-
The MondoA-dependent TXNIP/GDF15 axis predicts oxaliplatin response in colorectal adenocarcinomas
Authors: Deng, J;Pan, T;Wang, D;Hong, Y;Liu, Z;Zhou, X;An, Z;Li, L;Alfano, G;Li, G;Dolcetti, L;Evans, R;Vicencio, JM;Vlckova, P;Chen, Y;Monypenny, J;Gomes, CAC;Weitsman, G;Ng, K;McCarthy, C;Yang, X;Hu, Z;Porter, JC;Tape, CJ;Yin, M;Wei, F;Rodriguez-Justo, M;Zhang, J;Tejpar, S;Beatson, R;Ng, T;
EMBO molecular medicine
Species: Human
Sample Types: Cell Culture Supernates
-
A unique serum IgG glycosylation signature predicts development of Crohn's disease and is associated with pathogenic antibodies to mannose glycan
Authors: Gaifem, J;Rodrigues, CS;Petralia, F;Alves, I;Leite-Gomes, E;Cavadas, B;Dias, AM;Moreira-Barbosa, C;Revés, J;Laird, RM;Novokmet, M;tambuk, J;Habazin, S;Turhan, B;Gümü?, ZH;Ungaro, R;Torres, J;Lauc, G;Colombel, JF;Porter, CK;Pinho, SS;
Nature immunology
Species: Human
Sample Types: Cell Culture Supernates
-
Interferon subverts an AHR-JUN axis to promote CXCL13+ T cells in lupus
Authors: Law, C;Wacleche, VS;Cao, Y;Pillai, A;Sowerby, J;Hancock, B;Horisberger, A;Bracero, S;Skidanova, V;Li, Z;Adejoorin, I;Dillon, E;Benque, IJ;Nunez, DP;Simmons, DP;Keegan, J;Chen, L;Baker, T;Brohawn, PZ;Al-Mossawi, H;Hao, LY;Jones, B;Rao, N;Qu, Y;Alves, SE;Accelerating Medicines Partnership: RA/SLE Network, ;Jonsson, AH;Shaw, KS;Vleugels, RA;Massarotti, E;Costenbader, KH;Brenner, MB;Lederer, JA;Hultquist, JF;Choi, J;Rao, DA;
Nature
Species: Human
Sample Types: Cell Culture Supernates
-
GABA-mediated inhibition of human CD4+ T cell functions is enhanced by insulin but impaired by high glucose levels
Authors: Jin, Z;Hammoud, H;Bhandage, AK;Korol, SV;Trujeque-Ramos, O;Koreli, S;Gong, Z;Chowdhury, AI;Sandbaumhüter, FA;Jansson, ET;Lindsay, RS;Christoffersson, G;Andrén, PE;Carlsson, PO;Bergsten, P;Kamali-Moghaddam, M;Birnir, B;
EBioMedicine
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical and parasitological features of Leishmania infection among gold miners in the Oiapoque basin, an international Brazil-French Guiana border
Authors: Mosquera Atehortua, P;Figueira da Silva, A;Mafra, L;Almeida-da-Silveira, S;De Mello, CX;Gomes Albuquerque, H;André Boaventura de Carvalho, L;Hureau-Mutricy, L;Douine, M;Maria Da-Cruz, A;C Suárez-Mutis, M;Gomes-Silva, A;
PLoS neglected tropical diseases
Species: Human
Sample Types: Plasma
-
Reduced Tolerogenic Program Death-Ligand 1-Expressing Conventional Type 1 Dendritic Cells Are Associated with Rapid Decline in Chronic Obstructive Pulmonary Disease
Authors: Chen, KY;Sun, WL;Wu, SM;Feng, PH;Lin, CF;Chen, TT;Lu, YH;Ho, SC;Chen, YH;Lee, KY;
Cells
Species: Human
Sample Types: Cell Culture Supernates
-
Hotspot DNA Methyltransferase 3A (DNMT3A) and Isocitrate Dehydrogenase 1 and 2 (IDH1/2) Mutations in Acute Myeloid Leukemia and Their Relevance as Targets for Immunotherapy
Authors: Struckman, NE;de Jong, RCM;Honders, MW;Smith, SI;van der Lee, DI;Koutsoumpli, G;de Ru, AH;Mikesch, JH;van Veelen, PA;Falkenburg, JHF;Griffioen, M;
Biomedicines
Species: Human
Sample Types: Cell Culture Supernates
-
Association of the Reduced Levels of Monocyte Chemoattractant Protein-1 with Herpes Zoster in Rheumatoid Arthritis Patients Treated with Janus Kinase Inhibitors in a Single-Center Cohort
Authors: Chen, PK;Chen, YM;Chen, HH;Liao, TL;Chang, SH;Yeo, KJ;Huang, PH;Chen, DY;
Microorganisms
Species: Human
Sample Types: Plasma
-
Soluble CD27 is an intrathecal biomarker of T-cell-mediated lesion activity in multiple sclerosis
Authors: Cencioni, MT;Magliozzi, R;Palmisano, I;Suwan, K;Mensi, A;Fuentes-Font, L;Villar, LM;Fernández-Velasco, JI;Migallón, NV;Costa-Frossard, L;Monreal, E;Ali, R;Romozzi, M;Mazarakis, N;Reynolds, R;Nicholas, R;Muraro, PA;
Journal of neuroinflammation
Species: Human
Sample Types: Cell Culture Supernates, CSF
-
Effects of Combinatory In Vitro Treatment with Immune Checkpoint Inhibitors and Cytarabine on the Anti-Cancer Immune Microenvironment in De Novo AML Patients
Authors: Bo?kun, ?;Starosz, A;Kr?towska-Grunwald, A;Wasiluk, T;Walewska, A;Wierzbowska, A;Moniuszko, M;Grubczak, K;
Cancers
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-?3 is induced by Leishmania donovani and can inhibit parasite growth in cell line models but not in the mouse model, while it shows a significant association with leishmaniasis in humans
Authors: De, M;Sukla, S;Bharatiya, S;Keshri, S;Roy, DG;Roy, S;Dutta, D;Saha, S;Ejazi, SA;Ravichandiran, V;Ali, N;Chatterjee, M;Chinnaswamy, S;
Infection and immunity
Species: Human
Sample Types: Cell Culture Supernates
-
Suppression of NK Cell Activation by JAK3 Inhibition: Implication in the Treatment of Autoimmune Diseases
Authors: Wu, WC;Shiu, C;Tong, TK;Leung, SO;Hui, CW;
Journal of immunology research
Species: Human
Sample Types: Cell Culture Supernates
-
Neutralizing tumor-related inflammation and reprogramming of cancer-associated fibroblasts by Curcumin in breast cancer therapy
Authors: Jalilian, E;Abolhasani-Zadeh, F;Afgar, A;Samoudi, A;Zeinalynezhad, H;Langroudi, L;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Acid ceramidase regulates innate immune memory
Authors: Rother, N;Yanginlar, C;Prévot, G;Jonkman, I;Jacobs, M;van Leent, MMT;van Heck, J;Matzaraki, V;Azzun, A;Morla-Folch, J;Ranzenigo, A;Wang, W;van der Meel, R;Fayad, ZA;Riksen, NP;Hilbrands, LB;Lindeboom, RGH;Martens, JHA;Vermeulen, M;Joosten, LAB;Netea, MG;Mulder, WJM;van der Vlag, J;Teunissen, AJP;Duivenvoorden, R;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
A Comparison of the Antitumor Efficacy of Novel Multi-Specific Tribodies with Combinations of Approved Immunomodulatory Antibodies
Authors: Manna, L;Rapuano Lembo, R;Yoshioka, A;Nakamura, K;Passariello, M;De Lorenzo, C;
Cancers
Species: Human
Sample Types: Cell Culture Supernates
-
Ventilatory support and inflammatory peptides in hospitalised patients with COVID-19: A prospective cohort trial
Authors: Gysan, MR;Milacek, C;Bal, C;Zech, A;Brugger, J;Milos, RI;Antoniewicz, L;Idzko, M;Gompelmann, D;
PloS one
Species: Human
Sample Types: Serum
-
Serum interleukin-6, procalcitonin, and C-reactive protein at hospital admission can identify patients at low risk for severe COVID-19 progression
Authors: Zobel, CM;Wenzel, W;Krüger, JP;Baumgarten, U;Wagelöhner, T;Neumann, N;Foroutan, B;Müller, R;Müller, A;Rauschning, D;Schü beta ler, M;Scheit, L;Weinreich, F;Oltmanns, K;Keidel, F;Koch, M;Spethmann, S;Schreiner, M;
Frontiers in microbiology
Species: Human
Sample Types: Serum
-
NK Receptors Replace CD28 As the Dominant Source of Signal 2 for Cognate Recognition of Cancer Cells by TAA-specific Effector CD8+ T Cells
Authors: Dong, B;Obermajer, N;Tsuji, T;Matsuzaki, J;Bonura, C;Withers, H;Long, M;Chavel, C;Olejniczak, SH;Minderman, H;Edwards, RP;Storkus, WJ;Romero, P;Kalinski, P;
Research square
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical Staphylococcus aureus inhibits human T-cell activity through interaction with the PD-1 receptor
Authors: Mellergaard, M;Skovbakke, SL;Jepsen, SD;Panagiotopoulou, N;Hansen, ABR;Tian, W;Lund, A;Høgh, RI;Møller, SH;Guérillot, R;Hayes, AS;Erikstrup, LT;Andresen, L;Peleg, AY;Larsen, AR;Stinear, TP;Handberg, A;Erikstrup, C;Howden, BP;Goletz, S;Frees, D;Skov, S;
mBio
Species: Human
Sample Types: Cell Culture Supernates
-
Cytotoxic immune cells do not affect TDP-43 and p62 sarcoplasmic aggregation but influence TDP-43 localisation
Authors: McCord, B;Day, RM;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Social Determinants modulate NK cell activity via obesity, LDL, and DUSP1 signaling
Authors: Baumer, Y;Singh, K;Baez, AS;Gutierrez-Huerta, CA;Chen, L;Igboko, M;Turner, BS;Yeboah, JA;Reger, RN;Ortiz-Whittingham, LR;Bleck, CKE;Mitchell, VM;Collins, BS;Pirooznia, M;Dagur, PK;Allan, DSJ;Muallem-Schwartz, D;Childs, RW;Powell-Wiley, TM;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Parkin deficiency suppresses antigen presentation to promote tumor immune evasion and immunotherapy resistance
Authors: Perales-Linares, R;Leli, NM;Mohei, H;Beghi, S;Rivera, OD;Kostopoulos, N;Giglio, A;George, SS;Uribe-Herranz, M;Costabile, F;Pierini, S;Pustylnikov, S;Skoufos, G;Barash, Y;Hatzigeorgiou, AG;Koumenis, C;Maity, A;Lotze, MT;Facciabene, A;
Cancer research
Species: Human
Sample Types: Cell Culture Supernates
-
Development of a Novel CD26-Targeted Chimeric Antigen Receptor T-Cell Therapy for CD26-Expressing T-Cell Malignancies
Authors: Kobayashi, E;Kamihara, Y;Arai, M;Wada, A;Kikuchi, S;Hatano, R;Iwao, N;Susukida, T;Ozawa, T;Adachi, Y;Kishi, H;Dang, NH;Yamada, T;Hayakawa, Y;Morimoto, C;Sato, T;
Cells
Species: Human
Sample Types: Cell Culture Supernates
-
The influence of calcitriol and methylprednisolone on podocytes function in minimal change disease in vitro model
Authors: Grubczak, K;Starosz, A;Makowska, B;Parfienowicz, Z;Kr?towska, M;Naumnik, B;Moniuszko, M;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernatants
-
Dimethyl itaconate induces long-term innate immune responses and confers protection against infection
Authors: Ferreira, AV;Kostidis, S;Groh, LA;Koeken, VACM;Bruno, M;Baydemir, I;Kilic, G;Bulut, Ö;Andriopoulou, T;Spanou, V;Synodinou, KD;Gkavogianni, T;Moorlag, SJCFM;Charlotte de Bree, L;Mourits, VP;Matzaraki, V;Koopman, WJH;van de Veerdonk, FL;Renieris, G;Giera, M;Giamarellos-Bourboulis, EJ;Novakovic, B;Domínguez-Andrés, J;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Omega-3 polyunsaturated fatty acids (n-3 PUFAs), somatic and fatigue symptoms in cardiovascular diseases comorbid major depressive disorder (MDD): A randomized controlled trial
Authors: Chang, JP;Chang, SS;Chen, HT;Chien, YC;Yang, HT;Huang, SY;Tseng, PT;Chang, CH;Galecki, P;Su, KP;
Brain, behavior, and immunity
Species: Human
Sample Types: Plasma
-
CCX559 is a potent, orally-administered small molecule PD-L1 inhibitor that induces anti-tumor immunity
Authors: Sullivan, KMC;Vilalta, M;Ertl, LS;Wang, Y;Dunlap, C;Ebsworth, K;Zhao, BN;Li, S;Zeng, Y;Miao, Z;Fan, P;Mali, V;Lange, C;McMurtrie, D;Yang, J;Lui, R;Scamp, R;Chhina, V;Kumamoto, A;Yau, S;Dang, T;Easterday, A;Liu, S;Miao, S;Charo, I;Schall, TJ;Zhang, P;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Chimeric Newcastle Disease Virus Vectors Expressing Human IFN-gamma Mediate Target Immune Responses and Enable Multifaceted Treatments
Authors: RM Soliman, K Nishioka, T Daidoji, O Noyori, T Nakaya
Biomedicines, 2023-02-04;11(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Autofluorescence lifetime imaging classifies human lymphocyte activation and subtype
Authors: RL Schmitz, KE Tweed, P Rehani, K Samimi, J Riendeau, I Jones, EM Maly, EC Guzman, MH Forsberg, A Shahi, CM Capitini, AJ Walsh, MC Skala
bioRxiv : the preprint server for biology, 2023-01-23;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
PD-1highCXCR5-CD4+ peripheral helper T�cells promote CXCR3+ plasmablasts in human acute viral infection
Authors: H Asashima, S Mohanty, M Comi, WE Ruff, KB Hoehn, P Wong, J Klein, C Lucas, I Cohen, S Coffey, N Lele, L Greta, K Raddassi, O Chaudhary, A Unterman, B Emu, SH Kleinstein, RR Montgomery, A Iwasaki, CS Dela Cruz, N Kaminski, AC Shaw, DA Hafler, TS Sumida
Cell Reports, 2023-01-02;0(0):111895.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-GRP-R monoclonal antibody antitumor therapy against neuroblastoma
Authors: J Qiao, J Liu, JC Jacobson, RA Clark, S Lee, L Liu, Z An, N Zhang, DH Chung
PLoS ONE, 2022-12-16;17(12):e0277956.
Species: Human
Sample Types: Cell Culture Supernates
-
Genome-wide gain-of-function screening characterized lncRNA regulators for tumor immune response
Authors: Y Wang, Y Zhao, W Guo, GS Yadav, C Bhaskarla, Z Wang, X Wang, S Li, Y Wang, Y Chen, D Pattarayan, W Xie, S Li, B Lu, US Kammula, M Zhang, D Yang
Science Advances, 2022-12-07;8(49):eadd0005.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of exogenous IL-37 on immune cells from a patient carrying a potential IL37 loss-of-function variant: A case study
Authors: LU Teufel, CI van der Ma, V Klück, A Simons, A Hoischen, V Vernimmen, LAB Joosten, RJW Arts
Cytokine, 2022-12-05;162(0):156102.
Species: Human
Sample Types: Cell Culture Supernates
-
Large libraries of single-chain trimer peptide-MHCs enable rapid antigen-specific CD8+ T cell discovery and analysis
Authors: J Heath, W Chour, J Choi, J Xie, M Chaffee, T Schmitt, K Finton, D Delucia, A Xu, Y Su, D Chen, R Zhang, D Yuan, S Hong, A Ng, J Butler, R Edmark, L Jones, K Murray, S Peng, G Li, R Strong, J Lee, J Goldman, P Greenberg
Research square, 2022-11-18;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
CD169+ Monocyte and Regulatory T Cell Subsets Are Associated with Disease Activity in Rheumatoid Arthritis
Authors: AJ Eakin, T Ahmed, CM McGeough, S Drain, HD Alexander, GD Wright, PV Gardiner, D Small, AJ Bjourson, DS Gibson
Journal of personalized medicine, 2022-11-09;12(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
T Lymphocyte Serotonin 5-HT7 Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients
Authors: F Reverchon, C Guillard, L Mollet, P Auzou, D Gosset, F Madouri, A Valéry, A Menuet, C Ozsancak, M Pallix-Guy, S Morisset-L
Biomedicines, 2022-09-27;10(10):.
Species: Human
Sample Types: Plasma
-
Co-expression IL-15 receptor alpha with IL-15 reduces toxicity via limiting IL-15 systemic exposure during CAR-T immunotherapy
Authors: Y Zhang, Q Zhuang, F Wang, C Zhang, C Xu, A Gu, WH Zhong, Y Hu, X Zhong
Oncogene, 2022-09-27;20(1):432.
Species: Human
Sample Types: Cell Culture Supernates
-
Exercise training-induced changes in immunometabolic markers in youth badminton athletes
Authors: FE Rossi, AJ Maldonado, JM Cholewa, SLG Ribeiro, CA de Araújo, C Figueiredo, T Reichel, K Krüger, FS Lira, LG Minuzzi
Scientific Reports, 2022-09-15;12(1):15539.
Species: Human
Sample Types: Plasma
-
Generation and Functional Characterization of PLAP CAR-T Cells against Cervical Cancer Cells
Authors: V Yekehfalla, S Pahlavanne, A Sayadmanes, Z Momtahan, B Ma, M Basiri
Biomolecules, 2022-09-14;12(9):.
Species: Human
Sample Types: Cell Culture Supernatants
-
Human Allogeneic Liver-Derived Progenitor Cells Significantly Improve NAFLD Activity Score and Fibrosis in Late-Stage NASH Animal Model
Authors: M Najimi, S Michel, MM Binda, K Gellynck, N Belmonte, G Mazza, N Gordillo, Y Vainilovic, E Sokal
Cells, 2022-09-13;11(18):.
Species: Human
Sample Types: Cell Culture Supernates
-
Microcalorimetric Investigations of Reversible Staphylococcal Enterotoxin Unfolding
Authors: SC Berry, OA Triplett, LR Yu, ME Hart, LS Jackson, WH Tolleson
Toxins, 2022-08-15;14(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
SARS-CoV-2 Spike Does Not Possess Intrinsic Superantigen-like Inflammatory Activity
Authors: C Amormino, V Tedeschi, G Paldino, S Arcieri, MT Fiorillo, A Paiardini, L Tuosto, M Kunkl
Cells, 2022-08-15;11(16):.
Species: Human
Sample Types: Cell Culture Supernates
-
Lithocholic acid inhibits dendritic cell activation by reducing intracellular glutathione via TGR5 signaling
Authors: J Hu, Y Zhang, S Yi, C Wang, X Huang, S Pan, J Yang, G Yuan, S Tan, H Li
International journal of biological sciences, 2022-07-11;18(11):4545-4559.
Species: Human
Sample Types: Cell Culture Supernates
-
Activated CLL cells regulate IL17F producing Th17 cells in miR155 dependent and outcome specific manners
Authors: B Jung, G Ferrer, PY Chiu, R Aslam, A Ng, F Palacios, M Wysota, M Cardillo, JE Kolitz, SL Allen, JC Barrientos, KR Rai, N Chiorazzi, B Sherry
JCI Insight, 2022-06-22;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
"HIIT the Inflammation": Comparative Effects of Low-Volume Interval Training and Resistance Exercises on Inflammatory Indices in Obese Metabolic Syndrome Patients Undergoing Caloric Restriction
Authors: D Reljic, W Dieterich, HJ Herrmann, MF Neurath, Y Zopf
Nutrients, 2022-05-10;14(10):.
Species: Human
Sample Types: Serum
-
Onchocerca volvulus-specific antibody and cellular responses in onchocerciasis patients treated annually with ivermectin for 30 years and exposed to parasite transmission in central Togo
Authors: SI Johanns, RG Gantin, B Wangala, K Komlan, WA Halatoko, M Banla, P Karabou, AJ Luty, H Schulz-Key, C Köhler, PT Soboslay
PloS Neglected Tropical Diseases, 2022-05-03;16(5):e0010340.
Species: Human
Sample Types: Cell Culture Supernates
-
Analysis of the Seasonal Fluctuation of gammadelta T Cells and Its Potential Relation with Vitamin D3
Authors: B Bernicke, N Engelbogen, K Klein, J Franzenbur, C Borzikowsk, C Peters, O Janssen, R Junker, R Serrano, D Kabelitz
Cells, 2022-04-26;11(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Novel Bi-Specific Immuno-Modulatory Tribodies Potentiate T Cell Activation and Increase Anti-Tumor Efficacy
Authors: M Passariell, A Yoshioka, K Takahashi, SI Hashimoto, R Rapuano Le, L Manna, K Nakamura, C De Lorenzo
International Journal of Molecular Sciences, 2022-03-23;23(7):.
Species: Human
Sample Types: cell culture supernatant
-
The presence of serum anti-SARS-CoV-2 IgA appears to protect primary health care workers from COVID-19
Authors: V Hennings, K Thörn, S Albinsson, C Lingblom, K Andersson, C Andersson, K Järbur, R Pullerits, M Idorn, SR Paludan, K Eriksson, C Wennerås
European Journal of Immunology, 2022-02-18;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Echinococcus multilocularis specific antibody, systemic cytokine, and chemokine levels, as well as antigen-specific cellular responses in patients with progressive, stable, and cured alveolar echinococcosis: A 10-year follow-up
Authors: B Grüner, L Peters, A Hillenbran, P Vo beta berg, J Schweiker, EG Rollmann, LH Rodriguez, J Blumhardt, S Burkert, P Kern, C Köhler, PT Soboslay
PloS Neglected Tropical Diseases, 2022-02-02;16(2):e0010099.
Species: Human
Sample Types: Cell Culture Supernates
-
Novel Combinations of Human Immunomodulatory mAbs Lacking Cardiotoxic Effects for Therapy of TNBC
Authors: C Vetrei, M Passariell, G Froechlich, R Rapuano Le, E Sasso, N Zambrano, C De Lorenzo
Cancers, 2021-12-27;14(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Soluble CD137 is a novel serum marker of liver cirrhosis in patients with hepatitis C and alcohol-associated disease etiology
Authors: K Weigand, G Peschel, J Grimm, K Luu, D Schacherer, R Wiest, M Müller, H Schwarz, C Buechler
European Journal of Immunology, 2021-12-26;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Pluripotent Stem Cell-Derived Hepatocytes Inhibit T Cell Proliferation In Vitro through Tryptophan Starvation
Authors: M Romano, R Elgueta, D McCluskey, AM Ortega-Pri, E Stolarczyk, F Dazzi, B Lucendo-Vi, J Meseguer-R, J Williams, G Fanelli, DC Hay, FM Watt, G Lombardi
Cells, 2021-12-22;11(1):.
Species: Human
Sample Types: Serum
-
Reduced Interleukin-17-Expressing Cells in Cutaneous Melanoma
Authors: A Tosi, L Nardinocch, ML Carbone, L Capriotti, E Pagani, S Mastroeni, C Fortes, F Scopelliti, C Cattani, F Passarelli, A Rosato, S D'Atri, CM Failla, A Cavani
Biomedicines, 2021-12-16;9(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Skin Substitute Preparation Method Induces Immunomodulatory Changes in Co-Incubated Cells through Collagen Modification
Authors: J Holl, C Pawlukiani, J Corton Rui, D Groth, K Grubczak, HR Hady, J Dadan, J Reszec, S Czaban, C Kowalewski, M Moniuszko, A Eljaszewic
Pharmaceutics, 2021-12-15;13(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cancer immune therapy with PD-1-dependent CD137 co-stimulation provides localized tumour killing without systemic toxicity
Authors: Y Qiao, Y Qiu, J Ding, N Luo, H Wang, X Ling, J Sun, Z Wu, Y Wang, Y Liu, F Guo, T Sun, W Shen, M Zhang, D Wu, B Chen, W Xu, X Wang
Nature Communications, 2021-11-04;12(1):6360.
Species: Human
Sample Types: Cell Culture Supernates
-
Targeting of Janus Kinases Limits Pro-Inflammatory but Also Immunosuppressive Circuits in the Crosstalk between Synovial Fibroblasts and Lymphocytes
Authors: N Yao, T Tretter, P Kvacskay, W Merkt, N Blank, HM Lorenz, LO Tykocinski
Biomedicines, 2021-10-08;9(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Discovery of a novel anti PD-L1 X TIGIT bispecific antibody for the treatment of solid tumors
Authors: Y Xiao, P Chen, C Luo, Z Xu, X Li, L Liu, L Zhao
Cancer treatment and research communications, 2021-09-27;29(0):100467.
Species: Human
Sample Types: Cell Culture Supernates
-
Endothelial-Derived Extracellular Vesicles Induce Cerebrovascular Dysfunction in Inflammation
Authors: D Roig-Carle, E Willms, RD Fontijn, S Martinez-P, I Mäger, HE de Vries, M Hirst, B Sharrack, DK Male, CA Hawkes, IA Romero
Pharmaceutics, 2021-09-21;13(9):.
Species: Human
Sample Types: Extracellular Vesicles
-
Functional antibody and T-cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: the CAPTURE study
Authors: A Fendler, L Au, S Shepherd, F Byrne, M Cerrone, L Boos, K Rzeniewicz, W Gordon, B Shum, C Gerard, B Ward, W Xie, A Schmitt, N Joharatnam, G Cornish, M Pule, L Mekkaoui, K Ng, E Carlyle, K Edmonds, LD Rosario, S Sarker, K Lingard, M Mangwende, L Holt, H Ahmod, R Stone, C Gomes, H Flynn, A Agua-Doce, P Hobson, S Caidan, M Howell, M Wu, R Goldstone, M Crawford, L Cubitt, H Patel, M Gavrielide, E Nye, A Snijders, J MacRae, J Nicod, F Gronthoud, R Shea, C Messiou, D Cunningham, I Chau, N Starling, N Turner, L Welsh, N van As, R Jones, J Droney, S Banerjee, K Tatham, S Jhanji, M O'Brien, O Curtis, K Harrington, S Bhide, J Bazin, A Robinson, C Stephenson, T Slattery, Y Khan, Z Tippu, I Leslie, S Gennatas, A Okines, A Reid, K Young, A Furness, L Pickering, S Gandhi, S Gamblin, C Swanton, E Nicholson, S Kumar, N Yousaf, K Wilkinson, A Swerdlow, R Harvey, G Kassiotis, J Larkin, R Wilkinson, S Turajlic
Research square, 2021-09-20;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
alphabeta-T Cells Engineered to Express gammadelta-T Cell Receptors Can Kill Neuroblastoma Organoids Independent of MHC-I Expression
Authors: JGM Strijker, R Pscheid, E Drent, JJF van der Ho, B Koopmans, K Ober, SR van Hooff, WM Kholosy, AM Cornel, C Coomans, A Bisso, MM van Loenen, JJ Molenaar, J Wienke
Journal of personalized medicine, 2021-09-17;11(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Kras-driven intratumoral heterogeneity triggers infiltration of M2 polarized macrophages via the circHIPK3/PTK2 immunosuppressive circuit
Authors: T Katopodi, S Petanidis, K Domvri, P Zarogoulid, D Anestakis, C Charalampi, D Tsavlis, C Bai, H Huang, L Freitag, W Hohenforst, D Matthaios, K Porpodis
Scientific Reports, 2021-07-29;11(1):15455.
Species: Human
Sample Types: Cell Culture Supernates
-
Monocyte-dependent co-stimulation of cytokine induction in human gammadelta T cells by TLR8 RNA ligands
Authors: R Serrano, C Coch, C Peters, G Hartmann, D Wesch, D Kabelitz
Scientific Reports, 2021-07-27;11(1):15231.
Species: Human
Sample Types: Cell Culture Supernates
-
T-cell responses to SARS-CoV-2 in multiple sclerosis patients treated with ocrelizumab healed from COVID-19 with absent or low anti-spike antibody titers
Authors: M Iannetta, D Landi, G Cola, V Malagnino, E Teti, D Fraboni, F Buccisano, S Grelli, L Coppola, L Campogiani, M Andreoni, GA Marfia, L Sarmati
Multiple sclerosis and related disorders, 2021-07-21;55(0):103157.
Species: Human
Sample Types: Cell Culture Supernates
-
A scDb-based trivalent bispecific antibody for T-cell-mediated killing of HER3-expressing cancer cells
Authors: N Aschmoneit, S Steinlein, L Kühl, O Seifert, RE Kontermann
Scientific Reports, 2021-07-06;11(1):13880.
Species: Human
Sample Types: Cell Culture Supernates
-
Biomarkers for Comorbidities Modulate the Activity of T-Cells in COPD
Authors: K Jamal Jame, WJ Gallert, SD Yanik, S Panek, J Kronsbein, D Jungck, A Koch, J Knobloch
International Journal of Molecular Sciences, 2021-07-02;22(13):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cord blood and amniotic membrane extract eye drop preparations display immune-suppressive and regenerative properties
Authors: D Samarkanov, S Cox, D Hernandez, L Rodriguez, ML Pérez, A Madrigal, A Vilarrodon, S Querol, RP Casaroli-M
Scientific Reports, 2021-07-02;11(1):13754.
Species: Human
Sample Types: Cell Culture Supernates
-
Novel immunomodulatory properties of low dose cytarabine entrapped in a mannosylated cationic liposome
Authors: AL Martel, NL Fraleigh, E Picard, JD Lewicky, G Pawelec, H Lee, GW Ma, L Mousavifar, R Roy, HT Le
International journal of pharmaceutics, 2021-07-01;0(0):120849.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of monoclonal antibodies against human renal glomerular endothelial cells in lupus nephritis that induce endothelial interferon-alpha production
Authors: YC Hu, IJ Tsai, HY Hsu, BL Chiang, YH Yang
Arthritis Research & Therapy, 2021-06-16;23(1):171.
Species: Human
Sample Types: Cell Culture Supernates
-
P32-specific CAR T cells with dual antitumor and antiangiogenic therapeutic potential in gliomas
Authors: L Rousso-Noo, I Mastandrea, S Talmor, T Waks, A Globerson, M Haugas, T Teesalu, L Alvarez-Va, Z Eshhar, D Friedmann-
Nature Communications, 2021-06-14;12(1):3615.
Species: Human
Sample Types: Cell Culture Supernates
-
Bacterial genotoxins induce T�cell senescence
Authors: SL Mathiasen, L Gall-Mas, IS Pateras, SDP Theodorou, MRJ Namini, MB Hansen, OCB Martin, CK Vadivel, K Ntostoglou, D Butter, M Givskov, C Geisler, AN Akbar, VG Gorgoulis, T Frisan, N Ødum, T Krejsgaard
Cell Reports, 2021-06-08;35(10):109220.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunomodulatory mAbs as Tools to Investigate on Cis-Interaction of PD-1/PD-L1 on Tumor Cells and to Set Up Methods for Early Screening of Safe and Potent Combinatorial Treatments
Authors: C Vetrei, M Passariell, G Froechlich, R Rapuano Le, N Zambrano, C De Lorenzo
Cancers, 2021-06-08;13(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
A Rational Designed Novel Bispecific Antibody for the Treatment of GBM
Authors: R Sun, Y Zhou, L Han, Z Pan, J Chen, H Zong, Y Bian, H Jiang, B Zhang, J Zhu
Biomedicines, 2021-06-03;9(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
Beneficial Effects of Mineralocorticoid Receptor Pathway Blockade against Endothelial Inflammation Induced by SARS-CoV-2 Spike Protein
Authors: E Jover, L Matilla, M Garaikoetx, A Fernández-, P Muntendam, F Jaisser, P Rossignol, N López-Andr
Biomedicines, 2021-06-03;9(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
NLRP1 acts as a negative regulator of Th17 cell programming in mice and humans with autoimmune diabetes
Authors: FRC Costa, JA Leite, DM Rassi, JF da Silva, J Elias-Oliv, JB Guimarães, MC Foss-Freit, NOS Câmara, A Pontillo, RC Tostes, JS Silva, D Carlos
Cell Reports, 2021-05-25;35(8):109176.
Species: Human
Sample Types: Serum
-
Severe T cell hyporeactivity in ventilated COVID-19 patients correlates with prolonged virus persistence and poor outcomes
Authors: K Renner, T Schwittay, S Chaabane, J Gottschlin, C Müller, C Tiefenböck, JN Salewski, F Winter, S Buchtler, S Balam, MV Malferthei, M Lubnow, D Lunz, B Graf, F Hitzenbich, F Hanses, H Poeck, M Kreutz, E Orsó, R Burkhardt, T Niedermair, C Brochhause, A Gessner, B Salzberger, M Mack
Nature Communications, 2021-05-21;12(1):3006.
Species: Human
Sample Types: Cell Culture Supernates
-
Ex vivo modelling of PD-1/PD-L1 immune checkpoint blockade under acute, chronic, and exhaustion-like conditions of T-cell stimulation
Authors: A Roberts, L Bentley, T Tang, F Stewart, C Pallini, J Juvvanapud, GR Wallace, AJ Cooper, A Scott, D Thickett, ST Lugg, H Bancroft, B Hemming, C Ferris, G Langman, A Robinson, J Chapman, B Naidu, T Pinkney, GS Taylor, K Brock, Z Stamataki, CA Brady, SJ Curnow, J Gordon, O Qureshi, NM Barnes
Scientific Reports, 2021-02-17;11(1):4030.
Species: Human
Sample Types: Cell Culture Supernates
-
Food Intolerance of Unknown Origin: Caused by Mucosal Inflammation? A Pilot Study
Authors: W Dieterich, E Tietz, M Kohl, PC Konturek, T Rath, MF Neurath, Y Zopf
Clinical and Translational Gastroenterology, 2021-02-17;12(2):e00312.
Species: Human
Sample Types: Tissue Homogenates
-
New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response
Authors: RP Madeira, LM Dal'Mas Ro, P de Cássia, C Mady, BM Ianni, AC Torrecilha
Journal of Immunology Research, 2021-01-11;2021(0):6650670.
Species: Human
Sample Types: Cell Culture Supernates
-
Exploring the Role of C-C Motif Chemokine Ligand-2 Single Nucleotide Polymorphism in Pulmonary Tuberculosis: A Genetic Association Study from North India
Authors: SK Biswas, M Mittal, E Sinha, V Singh, N Arela, B Bajaj, PK Tiwari, VM Katoch, KK Mohanty
Journal of Immunology Research, 2020-12-16;2020(0):1019639.
Species: Human
Sample Types: Serum
-
Acute Conditioning of Antigen-Expanded CD8+ T Cells via the GSK3&beta-mTORC Axis Differentially Dictates Their Immediate and Distal Responses after Antigen Rechallenge
Authors: P Taborska, D Stakheev, H Svobodova, Z Strizova, J Bartunkova, D Smrz
Cancers, 2020-12-14;12(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical Response to the CD95-Ligand Inhibitor Asunercept Is Defined by a Pro-Inflammatory Serum Cytokine Profile
Authors: A Radujkovic, T Boch, F Nolte, D Nowak, C Kunz, A Gieffers, C Müller-Tid, P Dreger, WK Hofmann, T Luft
Cancers, 2020-12-08;12(12):.
Species: Human
Sample Types: Serum
-
Monocytes differentiated into macrophages and dendritic cells in the presence of human IFN-&lambda3 or IFN-&lambda4 show distinct phenotypes
Authors: M De, A Bhushan, S Chinnaswam
J Leukoc Biol, 2020-11-17;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Aromadendrin Inhibits T Cell Activation via Regulation of Calcium Influx and NFAT Activity
Authors: HS Lee, GS Jeong
Molecules, 2020-10-08;25(19):.
Species: Human
Sample Types: Cell Culture Supernates
-
An Antibody Targeting ICOS Increases Intratumoral Cytotoxic to Regulatory T-cell Ratio and Induces Tumor Regression
Authors: RCA Sainson, AK Thotakura, M Kosmac, G Borhis, N Parveen, R Kimber, J Carvalho, SJ Henderson, KL Pryke, T Okell, S O'Leary, S Ball, C Van Krinks, L Gamand, E Taggart, EJ Pring, H Ali, H Craig, VWY Wong, Q Liang, RJ Rowlands, M Lecointre, J Campbell, I Kirby, D Melvin, V Germaschew, E Oelmann, S Quaratino, M McCourt
Cancer Immunology Research, 2020-09-30;8(12):1568-1582.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-18 and Hematopoietic Recovery after Allogeneic Stem Cell Transplantation
Authors: A Radujkovic, L Kordelas, R Bogdanov, C Müller-Tid, DW Beelen, P Dreger, T Luft
Cancers (Basel), 2020-09-28;12(10):.
Species: Human
Sample Types: Serum
-
Lentiviral delivery of combinatorial CAR/CRISPRi circuit into human primary T cells is enhanced by TBK1/IKK? complex inhibitor BX795
Authors: L Li, Y Gao, R Srivastava, W Wang, Q Xiong, Z Fang, A Pelayo, C Denson, A Goswami, R Harari-Ste, Z Yang, L Weng, LS Qi, FM Marincola
Journal of Translational Medicine, 2020-09-23;18(1):363.
Species: Human
Sample Types: Cell Culture Supernates
-
Targeting PD-L1 in non-small cell lung cancer using CAR T cells
Authors: M Liu, X Wang, W Li, X Yu, P Flores-Vil, ZY Xu-Monette, L Li, M Zhang, KH Young, X Ma, Y Li
Oncogenesis, 2020-08-13;9(8):72.
Species: Human
Sample Types: Cell Culture Supernates
-
Isolation of Two Novel Human Anti-CTLA-4 mAbs with Intriguing Biological Properties on Tumor and NK Cells
Authors: M Passariell, C Vetrei, E Sasso, G Froechlich, C Gentile, AM D'Alise, N Zambrano, E Scarselli, A Nicosia, C De Lorenzo
Cancers (Basel), 2020-08-06;12(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cholesterol metabolism drives regulatory B cell IL-10 through provision of geranylgeranyl pyrophosphate
Authors: JA Bibby, HA Purvis, T Hayday, A Chandra, K Okkenhaug, S Rosenzweig, I Aksentijev, M Wood, HJ Lachmann, C Kemper, AP Cope, E Perucha
Nat Commun, 2020-07-08;11(1):3412.
Species: Human
Sample Types: Cell Culture Supernates
-
ADAM12 is a costimulatory molecule that determines Th1 cell fate and mediates tissue inflammation
Authors: Y Liu, R Bockermann, M Hadi, I Safari, B Carrion, M Kveiborg, S Issazadeh-
Cell. Mol. Immunol., 2020-06-22;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability
Authors: S Guedan, A Madar, V Casado-Med, C Shaw, A Wing, F Liu, RM Young, CH June, AD Posey
J. Clin. Invest., 2020-06-01;130(6):3087-3097.
Species: Human
Sample Types: Cell Culture Supernates
-
Persistent elevation of intrathecal pro-inflammatory cytokines leads to multiple sclerosis-like cortical demyelination and neurodegeneration
Authors: RE James, R Schalks, E Browne, I Eleftheria, CP Munoz, ND Mazarakis, R Reynolds
Acta Neuropathol Commun, 2020-05-12;8(1):66.
Species: Human
Sample Types: Cell Culture Supernates
-
Influence of Indoleamine-2,3-Dioxygenase and Its Metabolite Kynurenine on gammadelta T Cell Cytotoxicity against Ductal Pancreatic Adenocarcinoma Cells
Authors: H Jonescheit, HH Oberg, D Gonnermann, M Hermes, V Sulaj, C Peters, D Kabelitz, D Wesch
Cells, 2020-05-06;9(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Differentially Expressed Genes of Natural Killer Cells Can Distinguish Rheumatoid Arthritis Patients from Healthy Controls
Authors: NM Elemam, MY Hachim, S Hannawi, AA Maghazachi
Genes (Basel), 2020-04-30;11(5):.
Species: Human
Sample Types: Plasma
-
Insights into P-Glycoprotein Inhibitors: New Inducers of Immunogenic Cell Death
Authors: J Kopecka, M Godel, S Dei, R Giampietro, DC Belisario, M Akman, M Contino, E Teodori, C Riganti
Cells, 2020-04-22;9(4):.
Species: Human
Sample Types: Cell Culture Supernatants
-
Gaps in Study Design for Immune Parameter Research for Latent Tuberculosis Infection: A Systematic Review
Authors: M Herrera, C Vera, Y Keynan, ZV Rueda
J Immunol Res, 2020-04-21;2020(0):8074183.
Species: Human
Sample Types: Plasma
-
Transcriptional downregulation of MHC class I and melanoma de- differentiation in resistance to PD-1 inhibition
Authors: JH Lee, E Shklovskay, SY Lim, MS Carlino, AM Menzies, A Stewart, B Pedersen, M Irvine, S Alavi, JYH Yang, D Strbenac, RPM Saw, JF Thompson, JS Wilmott, RA Scolyer, GV Long, RF Kefford, H Rizos
Nat Commun, 2020-04-20;11(1):1897.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of BAY 1905254, an Immune Checkpoint Inhibitor Targeting the Immunoglobulin-Like Domain Containing Receptor 2 (ILDR2)
Authors: J Huetter, U Gritzan, I Gutcher, WD Doecke, MV Luetke-Eve, S Golfier, HG Roider, AL Frisk, J Hunter, A Pow, A Drake, Z Levine, O Levy, M Azulay, I Barbiro, G Cojocaru, I Vaknin, B Kreft, L Roese
Cancer Immunol Res, 2020-04-20;8(7):895-911.
Species: Human
Sample Types: Cell Culture Supernates
-
A Cross-Sectional Study Evaluating the Effects of Resistance Exercise on Inflammation and Neurotrophic Factors in Elderly Women with Obesity
Authors: HT Roh, SY Cho, WY So
J Clin Med, 2020-03-20;9(3):.
Species: Human
Sample Types: Serum
-
Activation of Human &gamma&delta T Cells: Modulation by Toll-Like Receptor 8 Ligands and Role of Monocytes
Authors: R Serrano, D Wesch, D Kabelitz
Cells, 2020-03-13;9(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of Human NK Cell-Derived Exosomes: Role of DNAM1 Receptor In Exosome-Mediated Cytotoxicity Against Tumor
Authors: ALD Pace, N Tumino, F Besi, C Alicata, LA Conti, E Munari, E Maggi, P Vacca, L Moretta
Cancers (Basel), 2020-03-12;12(3):.
Species: Human
Sample Types: Cell Culture Lysates
-
&gamma&delta T Cells Kill Plasmodium falciparum in a Granzyme- and Granulysin-Dependent Mechanism during the Late Blood Stage
Authors: MA Hernández-, K Happ, F Cattalani, A Wallimann, M Blanchard, I Fellay, B Scolari, N Lannes, S Mbagwu, B Fellay, L Filgueira, PY Mantel, M Walch
J. Immunol., 2020-02-17;204(7):1798-1809.
Species: Human
Sample Types: Cell Culture Supernates
-
Ipilimumab and Its Derived EGFR Aptamer-Based Conjugate Induce Efficient NK Cell Activation against Cancer Cells
Authors: M Passariell, S Camorani, C Vetrei, S Ricci, L Cerchia, C De Lorenzo
Cancers (Basel), 2020-02-01;12(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Human immune cells infiltrate the spinal cord and impair recovery after spinal cord injury in humanized mice
Authors: RS Carpenter, RR Jiang, FH Brennan, JCE Hall, MK Gottipati, S Niewiesk, PG Popovich
Sci Rep, 2019-12-13;9(1):19105.
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
43?kDa Glycoprotein (gp43) from Paracoccidioides brasiliensis Induced IL-17A and PGE2 Production by Human Polymorphonuclear Neutrophils: Involvement of TLR2 and TLR4
Authors: TP Gardizani, AM Della Cole, GG Romagnoli, R Puccia, APM Serezani, ÂMV de Campos, LA Dias-Melic
J Immunol Res, 2019-11-26;2019(0):1790908.
Species: Human
Sample Types: Cell Culture Supernates
-
Programmed cell death ligand-1-mediated enhancement of hexokinase 2 expression is inversely related to T-cell effector gene expression in non-small-cell lung cancer
Authors: S Kim, JY Jang, J Koh, D Kwon, YA Kim, JC Paeng, CY Ock, B Keam, M Kim, TM Kim, DS Heo, DH Chung, YK Jeon
J. Exp. Clin. Cancer Res., 2019-11-12;38(1):462.
Species: Human
Sample Types: Media
-
Replication of Genome-Wide Association Analysis Identifies New Susceptibility Loci at Long Noncoding RNA Regions for Vogt-Koyanagi-Harada Disease
Authors: J Qi, L Du, J Deng, Y Qin, G Su, S Hou, M Lv, Q Zhang, A Kijlstra, P Yang
Invest. Ophthalmol. Vis. Sci., 2019-11-01;60(14):4820-4829.
Species: Human
Sample Types: Cell Culture Supernates
-
Restricting Glycolysis Preserves T Cell Effector Functions and Augments Checkpoint Therapy
Authors: K Renner, C Bruss, A Schnell, G Koehl, HM Becker, M Fante, AN Menevse, N Kauer, R Blazquez, L Hacker, SM Decking, T Bohn, S Faerber, K Evert, L Aigle, S Amslinger, M Landa, O Krijgsman, EA Rozeman, C Brummer, PJ Siska, K Singer, S Pektor, M Miederer, K Peter, E Gottfried, W Herr, I Marchiq, J Pouyssegur, WR Roush, S Ong, S Warren, T Pukrop, P Beckhove, SA Lang, T Bopp, CU Blank, JL Cleveland, PJ Oefner, K Dettmer, M Selby, M Kreutz
Cell Rep, 2019-10-01;29(1):135-150.e9.
Species: Human
Sample Types: Cell Culture Supernates
-
MET Receptor Tyrosine Kinase Regulates the Expression of Co-Stimulatory and Co-Inhibitory Molecules in Tumor Cells and Contributes to PD-L1-Mediated Suppression of Immune Cell Function
Authors: HK Ahn, S Kim, D Kwon, J Koh, YA Kim, K Kim, DH Chung, YK Jeon
Int J Mol Sci, 2019-09-01;20(17):.
Species: Human
Sample Types: Cell Culture Supernates
-
The human glomerular endothelial cells are potent pro-inflammatory contributors in an in vitro model of lupus nephritis
Authors: P Dimou, RD Wright, KL Budge, A Midgley, SC Satchell, M Peak, MW Beresford
Sci Rep, 2019-06-06;9(1):8348.
Species: Human
Sample Types: Cell Culture Supernates
-
A rationally designed peptide antagonist of the PD-1 signaling pathway as an immunomodulatory agent for cancer therapy
Authors: PG Sasikumar, RK Ramachandr, S Adurthi, AA Dhudashia, S Vadlamani, K Vemula, S Vunnum, LK Satyam, DS Samiulla, K Subbarao, R Nair, R Shrimali, N Gowda, M Ramachandr
Mol. Cancer Ther., 2019-04-23;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of vimentin in modulating immune cell apoptosis and inflammatory responses in sepsis
Authors: L Su, P Pan, P Yan, Y Long, X Zhou, X Wang, R Zhou, B Wen, L Xie, D Liu
Sci Rep, 2019-04-05;9(1):5747.
Species: Human
Sample Types: Cell Culture Supernates
-
Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis
Authors: C Wei, C Yang, S Wang, D Shi, C Zhang, X Lin, Q Liu, R Dou, B Xiong
Mol. Cancer, 2019-03-30;18(1):64.
Species: Human
Sample Types: Cell Culture Supernates
-
In Vitro Induction of T Helper 17 Cells by Synergistic Activation of Human Monocyte-Derived Langerhans Cell-Like Cells with Bacterial Agonists
Authors: R Gramlich, E Aliahmadi, M Peiser
Int J Mol Sci, 2019-03-19;20(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
Large-scale secretome analyses unveil the superior immunosuppressive phenotype of umbilical cord stromal cells as compared to other adult mesenchymal stromal cells
Authors: A Islam, I Urbarova, JA Bruun, I Martinez-Z
Eur Cell Mater, 2019-02-20;37(0):153-174.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of a Novel Third-Generation Anti-CD24-CAR against Ovarian Cancer
Authors: R Klapdor, S Wang, M Morgan, T Dörk, U Hacker, P Hillemanns, H Büning, A Schambach
Int J Mol Sci, 2019-02-03;20(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-CD137 monoclonal antibody enhances trastuzumab-induced, natural killer cell-mediated cytotoxicity against pancreatic cancer cell lines with low human epidermal growth factor-like receptor 2 expression
Authors: T Masu, M Atsukawa, K Nakatsuka, M Shimizu, D Miura, T Arai, H Harimoto, C Kondo, K Kaneko, S Futagami, C Kawamoto, H Takahashi, K Iwakiri
PLoS ONE, 2018-12-31;13(12):e0200664.
Species: Human
Sample Types: Cell Culture Supernates
-
PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia
Authors: T Lopatina, E Favaro, C Grange, M Cedrino, A Ranghino, S Occhipinti, S Fallo, F Buffolo, DA Gaykalova, MM Zanone, R Romagnoli, G Camussi
Sci Rep, 2018-12-04;8(1):17458.
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of salivary cytokines in response to alcohol, tobacco and caffeine consumption: a pilot study
Authors: CC Sheth, RM López-Pedr, MDM Jovani-San, R González-M, V Veses
Sci Rep, 2018-11-12;8(1):16687.
Species: Human
Sample Types: Saliva
-
An Anti-Inflammatory Azaphenothiazine Inhibits Interferon ? Expression and CXCL10 Production in KERTr Cells
Authors: L Strzadala, A Fiedorowic, E Wysokinska, E Ziolo, M Grudzie?, M Jelen, K Pluta, B Morak-Mlod, M Zimecki, W Kalas
Molecules, 2018-09-24;23(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Utilizing ethacrynic acid and ciclopirox olamine in liver cancer
Authors: AM Al-Dali, H Weiher, IGH Schmidt-Wo
Oncol Lett, 2018-09-20;16(5):6854-6860.
Species: Human
Sample Types: Cell Culture Supernates
-
CLEC16A regulates splenocyte and NK cell function in part through MEK signaling
Authors: R Pandey, M Bakay, HS Hain, B Strenkowsk, BZB Elsaqa, JD Roizen, JA Kushner, JS Orange, H Hakonarson
PLoS ONE, 2018-09-18;13(9):e0203952.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasmodium falciparum Treated with Artemisinin-based Combined Therapy Exhibits Enhanced Mutation, Heightened Cortisol and TNF-? Induction
Authors: AO Idowu, S Bhattachar, S Gradus, W Oyibo, Z George, C Black, J Igietseme, AA Azenabor
Int J Med Sci, 2018-09-07;15(13):1449-1457.
Species: Human
Sample Types: Plasma
-
Disabled-2 (DAB2) Overexpression Inhibits Monocyte-Derived Dendritic Cells' Function in Vogt-Koyanagi-Harada Disease
Authors: S Yi, R Chang, J Hu, Y Qiu, Q Wang, Q Cao, G Yuan, G Su, C Zhou, Y Wang, A Kijlstra, P Yang
Invest. Ophthalmol. Vis. Sci., 2018-09-04;59(11):4662-4669.
Species: Human
Sample Types: Cell Culture Supernates
-
Interferon priming is essential for human CD34+ cell-derived plasmacytoid dendritic cell maturation and function
Authors: A Laustsen, RO Bak, C Krapp, L Kjær, JH Egedahl, CC Petersen, S Pillai, HQ Tang, N Uldbjerg, M Porteus, NR Roan, M Nyegaard, PW Denton, MR Jakobsen
Nat Commun, 2018-08-30;9(1):3525.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-15 and IFN-? signal through the ERK pathway to inhibit HCV replication, independent of type I IFN signaling
Authors: F Vahedi, AJ Lee, SE Collins, MV Chew, E Lusty, B Chen, A Dubey, CD Richards, JJ Feld, RS Russell, KL Mossman, AA Ashkar
Cytokine, 2018-06-19;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Modifying Enzymes are Elicited by ER Stress, Generating Epitopes that are Selectively Recognized by CD4+ T Cells in Patients With Type 1 Diabetes
Authors: ML Marre, JW McGinty, IT Chow, ME DeNicola, NW Beck, SC Kent, AC Powers, R Bottino, DM Harlan, CJ Greenbaum, WW Kwok, JD Piganelli, EA James
Diabetes, 2018-04-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Improving CART-Cell Therapy of Solid Tumors with Oncolytic Virus-Driven Production of a Bispecific T-cell Engager
Authors: A Wing, CA Fajardo, AD Posey, C Shaw, T Da, RM Young, R Alemany, CH June, S Guedan
Cancer Immunol Res, 2018-03-27;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Analysis of the association between Fc receptor family gene polymorphisms and ocular Beh�et's disease in Han Chinese
Authors: D Zhang, J Qin, L Li, G Su, G Huang, Q Cao, A Kijlstra, P Yang
Sci Rep, 2018-03-19;8(1):4850.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinically compliant spatial and temporal imaging of chimeric antigen receptor T-cells
Authors: N Emami-Shah, J Foster, R Kashani, P Gazinska, C Cook, J Sosabowski, J Maher, S Papa
Nat Commun, 2018-03-14;9(1):1081.
Species: Human
Sample Types: Cell Culture Supernates
-
An anti-TL1A antibody for the treatment of asthma and inflammatory bowel disease
Authors: AW Clarke, L Poulton, D Shim, D Mabon, D Butt, M Pollard, V Pande, J Husten, J Lyons, C Tian, AG Doyle
MAbs, 2018-03-05;0(0):1-43.
Species: Human
Sample Types: Plasma
-
Narrow Spectrum Kinase Inhibitors Demonstrate Promise for the Treatment of Dry Eye Disease and Other Ocular Inflammatory Disorders
Authors: S Hagan, MCT Fyfe, B Ofori-Frim, K Oliver, MR Foster, S Sirohi, Y Solanke, M Doughty, A Rowley, M Taylor, S Webber, CA Walshe
Invest. Ophthalmol. Vis. Sci., 2018-03-01;59(3):1443-1453.
Species: Human
Sample Types: Cell Culture Supernates
-
MicroRNA-374b inhibits liver cancer progression via down regulating programmed cell death-1 expression on cytokine-induced killer cells
Authors: F Huang, B Wang, J Zeng, S Sang, J Lei, Y Lu
Oncol Lett, 2018-02-05;15(4):4797-4804.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of Long Noncoding RNAs Polymorphisms With Ankylosing Spondylitis, Vogt-Koyanagi-Harada Disease, and Behcet's Disease
Authors: Y Yue, J Zhang, L Yang, S Liu, J Qi, Q Cao, C Zhou, Y Wang, A Kijlstra, P Yang, S Hou
Invest. Ophthalmol. Vis. Sci., 2018-02-01;59(2):1158-1166.
Species: Human
Sample Types: Cell Culture Supernates
-
TiO2 nanoparticles can selectively bind CXCL8 impacting on neutrophil chemotaxis
Authors: J Batt, M Milward, I Chapple, M Grant, H Roberts, O Addison
Eur Cell Mater, 2018-01-19;35(0):13-24.
-
Tumor-Derived Mesenchymal Stem Cells Use Distinct Mechanisms to Block the Activity of Natural Killer Cell Subsets
Authors: S Galland, J Vuille, P Martin, I Letovanec, A Caignard, G Fregni, I Stamenkovi
Cell Rep, 2017-09-19;20(12):2891-2905.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-18/IL-15/IL-12 synergy induces elevated and prolonged IFN-? production by ex vivo expanded NK cells which is not due to enhanced STAT4 activation
Authors: E Lusty, SM Poznanski, K Kwofie, TS Mandur, DA Lee, CD Richards, AA Ashkar
Mol. Immunol., 2017-06-20;88(0):138-147.
Species: Human
Sample Types: Cell Culture Supernates
-
TB lymphadenitis is associated with enhanced baseline and antigen - specific induction of Type 1 and Type 17 cytokines and reduced IL-1? and IL-18 at the site of infection
Authors: GR Kathamuthu, K Moideen, D Baskaran, VV Banurekha, D Nair, G Sekar, R Sridhar, B Vidyajayan, G Gajendrara, DK Parandhama, A Srinivasan, S Babu
Clin. Vaccine Immunol, 2017-05-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Chronic Toxoplasma gondii Infection Exacerbates Secondary Polymicrobial Sepsis
Authors: MC Souza, DM Fonseca, A Kanashiro, L Benevides, TS Medina, MS Dias, WA Andrade, G Bonfá, MAB Silva, A Gozzi, MC Borges, RT Gazzinelli, JC Alves-Filh, FQ Cunha, JS Silva
Front Cell Infect Microbiol, 2017-04-07;7(0):116.
Species: Human
Sample Types: Serum
-
Identification of an immunogenic neo-epitope encoded by mouse sarcoma using CXCR3 ligand mRNAs as sensors
Authors: K Fujii, Y Miyahara, N Harada, D Muraoka, M Komura, R Yamaguchi, H Yagita, J Nakamura, S Sugino, S Okumura, S Imoto, S Miyano, H Shiku
Oncoimmunology, 2017-03-20;6(5):e1306617.
Species: Human
Sample Types: Cell Culture Supernates
-
Suction blistering the lesional skin of vitiligo patients reveals useful biomarkers of disease activity
Authors: JP Strassner, M Rashighi, M Ahmed Refa, JM Richmond, JE Harris
J. Am. Acad. Dermatol, 2017-03-01;0(0):.
Species: Human
Sample Types: Blister Fluid
-
On the cytokine/chemokine network during Plasmodium vivax malaria: new insights to understand the disease
Authors: NS Hojo-Souza, DB Pereira, FS de Souza, TA de Oliveir, MS Cardoso, MS Tada, GM Zanini, DC Bartholome, RT Fujiwara, LL Bueno
Malar. J, 2017-01-24;16(1):42.
Species: Human
Sample Types: Plasma
-
Metabolic and adaptive immune responses induced in mice infected with tissue-dwelling nematode Trichinella zimbabwensis
Authors: S Mukaratirw
Open Vet J, 2016-11-04;6(3):178-184.
Species: Mouse
Sample Types: Serum
-
Reduced systemic and mycobacterial antigen-stimulated concentrations of IL-1? and IL-18 in tuberculous lymphadenitis
Cytokine, 2016-10-26;90(0):66-72.
Species: Human
Sample Types: Cell Culture Supernates
-
Acanthamoeba castellanii (Genotype T4) Stimulates the Production of Interleukin-10 as well as Pro-inflammatory Cytokines in THP-1 Cells, Human Peripheral Blood Mononuclear Cells and Human Monocyte-Derived Macrophages
Infect Immun, 2016-09-19;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Mumps Virus Induces Protein-Kinase-R-Dependent Stress Granules, Partly Suppressing Type III Interferon Production
PLoS ONE, 2016-08-25;11(8):e0161793.
Species: Human
Sample Types: Cell Culture Supernates
-
DNA Sensing via TLR-9 Constitutes a Major Innate Immunity Pathway Activated during Erythema Nodosum Leprosum
J Immunol, 2016-07-29;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Dual Inhibition of Rip2 and IRAK1/4 Regulates IL-1? and IL-6 in Sarcoidosis Alveolar Macrophages and Peripheral Blood Mononuclear Cells
J Immunol, 2016-07-11;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Bortezomib treatment sensitizes oncolytic HSV-1 treated tumors to NK cell immunotherapy
Clin Cancer Res, 2016-07-07;0(0):.
Species: Mouse
Sample Types: Serum
-
Analysis of receptor tyrosine kinase genetics identifies two novel risk loci in GAS6 and PROS1 in Beh�et's disease
Sci Rep, 2016-05-25;6(0):26662.
Species: Human
Sample Types: Cell Culture Supernates
-
UNC-45A Is a Nonmuscle Myosin IIA Chaperone Required for NK Cell Cytotoxicity via Control of Lytic Granule Secretion.
Authors: Iizuka Y, Cichocki F, Sieben A, Sforza F, Karim R, Coughlin K, Isaksson Vogel R, Gavioli R, McCullar V, Lenvik T, Lee M, Miller J, Bazzaro M
J Immunol, 2015-10-05;195(10):4760-70.
Species: Human
Sample Types: Cell Culture Supernates
-
Priming of Human Resting NK Cells by Autologous M1 Macrophages via the Engagement of IL-1beta, IFN-beta, and IL-15 Pathways.
Authors: Mattiola I, Pesant M, Tentorio P, Molgora M, Marcenaro E, Lugli E, Locati M, Mavilio D
J Immunol, 2015-08-14;195(6):2818-28.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-23 Receptor Gene Polymorphism May Enhance Expression of the IL-23 Receptor, IL-17, TNF-alpha and IL-6 in Behcet's Disease.
Authors: Jiang Z, Hennein L, Tao Y, Tao L
PLoS ONE, 2015-07-29;10(7):e0134632.
Species: Human
Sample Types: Cell Culture Supernates
-
An Autocrine Cytokine/JAK/STAT-Signaling Induces Kynurenine Synthesis in Multidrug Resistant Human Cancer Cells.
Authors: Campia I, Buondonno I, Castella B, Rolando B, Kopecka J, Gazzano E, Ghigo D, Riganti C
PLoS ONE, 2015-05-08;10(5):e0126159.
Species: Human
Sample Types: Cell Culture Supernates
-
B7H6-Specific Bispecific T Cell Engagers Lead to Tumor Elimination and Host Antitumor Immunity.
Authors: Wu M, Zhang T, Gacerez A, Coupet T, DeMars L, Sentman C
J Immunol, 2015-04-24;194(11):5305-11.
Species: Human
Sample Types: Cell Culture Supernates
-
B7H6-specific chimeric antigen receptors lead to tumor elimination and host antitumor immunity.
Authors: Wu M, Zhang T, DeMars L, Sentman C
Gene Ther, 2015-04-01;22(8):675-84.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model.
Authors: Guirado E, Mbawuike U, Keiser T, Arcos J, Azad A, Wang S, Schlesinger L
MBio, 2015-02-17;6(1):e02537-14.
Species: Human
Sample Types: Cell Culture Supernates
-
Phenotypic profiling of CD8(+) T cells during Plasmodium vivax blood-stage infection.
Authors: Hojo-Souza N, Pereira D, Passos L, Gazzinelli-Guimaraes P, Cardoso M, Tada M, Zanini G, Bartholomeu D, Fujiwara R, Bueno L
BMC Infect Dis, 2015-01-31;15(0):35.
Species: Human
Sample Types: Plasma
-
Specific targeting of a naturally presented osteosarcoma antigen, papillomavirus binding factor peptide, using an artificial monoclonal antibody.
Authors: Tsukahara T, Emori M, Murata K, Hirano T, Muroi N, Kyono M, Toji S, Watanabe K, Torigoe T, Kochin V, Asanuma H, Matsumiya H, Yamashita K, Himi T, Ichimiya S, Wada T, Yamashita T, Hasegawa T, Sato N
J Biol Chem, 2014-06-24;289(32):22035-47.
Species: Human
Sample Types: Cell Culture Supernates
-
Interferon-gamma production by human neutrophils upon stimulation by IL-12, IL-15 and IL-18 and challenge with Paracoccidioides brasiliensis.
Authors: Rodrigues D, Fernandes R, Balderramas H, Penitenti M, Bachiega T, Calvi S, Dias-Melicio L, Ikoma M, Soares A
Cytokine, 2014-06-14;69(1):102-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Human CD4+ T cell responses to the dog major allergen Can f 1 and its human homologue tear lipocalin resemble each other.
Authors: Liukko A, Kinnunen T, Rytkonen-Nissinen M, Kailaanmaki A, Randell J, Maillere B, Virtanen T
PLoS ONE, 2014-05-29;9(5):e98461.
Species: Human
Sample Types: Cell Culture Supernates
-
A functional variant of PTPN22 confers risk for Vogt-Koyanagi-Harada syndrome but not for ankylosing spondylitis.
Authors: Zhang Q, Qi J, Hou S, DU L, Yu H, Cao Q, Zhou Y, Liao D, Kijlstra A, Yang P
PLoS ONE, 2014-05-09;9(5):e96943.
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-gamma stimulates autophagy-mediated clearance of Burkholderia cenocepacia in human cystic fibrosis macrophages.
Authors: Assani K, Tazi M, Amer A, Kopp B
PLoS ONE, 2014-05-05;9(5):e96681.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of interleukin-1 receptor-associated kinases in Vogt-Koyanagi-Harada disease.
Authors: Sun M, Yang P, DU L, Yang Y, Ye J
PLoS ONE, 2014-04-01;9(4):e93214.
Species: Human
Sample Types: Cell Culture Supernates
-
Engagement of SLAMF2/CD48 prolongs the time frame of effective T cell activation by supporting mature dendritic cell survival.
Authors: Kis-Toth K, Tsokos G
J Immunol, 2014-03-26;192(9):4436-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Natural killer cell-mediated shedding of ULBP2.
Authors: Wang, Ruipeng, Sun, Peter D
PLoS ONE, 2014-03-10;9(3):e91133.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunoepidemiological profiling of onchocerciasis patients reveals associations with microfilaria loads and ivermectin intake on both individual and community levels.
Authors: Arndts, Kathrin, Specht, Sabine, Debrah, Alexande, Tamarozzi, Francesc, Klarmann Schulz, Ute, Mand, Sabine, Batsa, Linda, Kwarteng, Alexande, Taylor, Mark, Adjei, Ohene, Martin, Coralie, Layland, Laura E, Hoerauf, Achim
PLoS Negl Trop Dis, 2014-02-20;8(2):e2679.
Species: Human
Sample Types: Cell Culture Supernates
-
The aryl hydrocarbon receptor is functionally upregulated early in the course ofvhuman T-cell activation.
Authors: Prigent L, Robineau M, Jouneau S, Morzadec C, Louarn L, Vernhet L, Fardel O, Sparfel L
Eur J Immunol, 2014-02-18;44(5):1330-40.
Species: Human
Sample Types: Cell Culture Supernates
-
CD137 ligand signalling induces differentiation of primary acute myeloid leukaemia cells.
Authors: Cheng K, Wong S, Linn Y, Ho L, Chng W, Schwarz H
Br J Haematol, 2014-01-15;165(1):134-44.
Species: Human
Sample Types: Cell Culture Supernates
-
Activin A as a mediator of NK-dendritic cell functional interactions.
Authors: Seeger P, Bosisio D, Parolini S, Badolato R, Gismondi A, Santoni A, Sozzani S
J Immunol, 2014-01-06;192(3):1241-8.
Species: Human
Sample Types: Cell Culture Supernates
-
TNF-alpha blockade induces IL-10 expression in human CD4+ T cells.
Authors: Evans H, Roostalu U, Walter G, Gullick N, Frederiksen K, Roberts C, Sumner J, Baeten D, Gerwien J, Cope A, Geissmann F, Kirkham B, Taams L
Nat Commun, 2014-01-01;5(0):3199.
Species: Human
Sample Types: Cell Culture Supernates
-
CD137L-stimulated dendritic cells are more potent than conventional dendritic cells at eliciting cytotoxic T-cell responses.
Authors: Harfuddin Z, Kwajah S, Chong Nyi Sim A, Macary P, Schwarz H
Oncoimmunology, 2013-11-11;2(11):e26859.
Species: Human
Sample Types: Cell Culture Supernates
-
T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy.
Authors: Yu F, Wang X, Guo Z, Bartlett D, Gottschalk S, Song X
Mol Ther, 2013-10-17;22(1):102-11.
Species: Human
Sample Types: Cell Culture Supernates
-
Fcgamma receptor-induced soluble vascular endothelial growth factor receptor-1 (VEGFR-1) production inhibits angiogenesis and enhances efficacy of anti-tumor antibodies.
Authors: Justiniano S, Elavazhagan S, Fatehchand K, Shah P, Mehta P, Roda J, Mo X, Cheney C, Hertlein E, Eubank T, Marsh C, Muthusamy N, Butchar J, Byrd J, Tridandapani S
J Biol Chem, 2013-07-31;288(37):26800-9.
Species: Human
Sample Types: Cell Culture Supernates
-
TLR3 impairment in human newborns.
Authors: Slavica L, Nordstrom I, Karlsson M, Valadi H, Kacerovsky M, Jacobsson B, Eriksson K
J Leukoc Biol, 2013-07-30;94(5):1003-11.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated adaptive immune responses are associated with latent infections of Wuchereria bancrofti.
Authors: Arndts K, Deininger S, Specht S, Klarmann U, Mand S, Adjobimey T, Debrah AY, Batsa L, Kwarteng A, Epp C, Taylor M, Adjei O, Layland LE, Hoerauf A
PLoS Negl Trop Dis, 2012-04-03;6(4):e1611.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist.
Authors: van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, Joosten LA, Van der Meer JW, Hao R, Kalabokis V, Dinarello CA
Proc. Natl. Acad. Sci. U.S.A., 2012-02-06;109(8):3001-5.
Species: Human
Sample Types: Cell Culture Supernates
-
Natural killer cell-produced IFN-gamma and TNF-alpha induce target cell cytolysis through up-regulation of ICAM-1.
Authors: Wang R, Jaw JJ, Jaw J, Stutzman NC, Stutzman N, Zou Z, Sun PD
J Leukoc Biol, 2011-11-01;91(2):299-309.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated serum osteopontin levels and genetic polymorphisms of osteopontin are associated with Vogt-Koyanagi-Harada disease.
Authors: Chu M, Yang P, Hu R, Hou S, Li F, Chen Y, Kijlstra A
Invest. Ophthalmol. Vis. Sci., 2011-09-09;52(10):7084-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum levels of Th17-related cytokines in Behcet disease patients after cataract surgery.
Authors: Jiang S, Liu X, Luo L, Qu B, Huang X, Lin Y, Ye S, Liu Y
Mol. Vis., 2011-05-31;17(0):1425-30.
Species: Human
Sample Types: Serum
-
NKG2D receptor regulates human effector T-cell cytokine production.
Authors: Barber A, Sentman CL
Blood, 2011-04-25;117(24):6571-81.
Species: Human
Sample Types: Cell Culture Supernates
-
Mutations of the von Hippel-Lindau gene confer increased susceptibility to natural killer cells of clear-cell renal cell carcinoma.
Authors: Perier A, Fregni G, Wittnebel S, Gad S, Allard M, Gervois N, Escudier B, Azzarone B, Caignard A
Oncogene, 2011-01-24;30(23):2622-32.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes.
Authors: Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, Nasorri F, Garcovich S, Traidl-Hoffmann C, Albanesi C, Cavani A
J. Immunol., 2010-03-31;184(9):4880-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunoregulation of autocrine prolactin: suppressing the expression of costimulatory molecules and cytokines in T lymphocytes by prolactin receptor knockdown.
Authors: Xu D, Lin L, Lin X
Cell. Immunol., 2010-03-02;263(1):71-8.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of vaccine timing on the efficacy of an acute eccentric exercise intervention on the immune response to an influenza vaccine in young adults.
Authors: Campbell JP, Edwards KM, Ring C
Brain Behav. Immun., 2009-10-08;24(2):236-42.
Species: Human
Sample Types: Plasma
-
Interferon-beta treatment in multiple sclerosis attenuates inflammatory gene expression through inducible activity of the phosphatase SHP-1.
Authors: Christophi GP, Panos M, Hudson CA, Tsikkou C, Mihai C, Mejico LJ, Jubelt B, Massa PT
Clin. Immunol., 2009-06-25;133(1):27-44.
Species: Human
Sample Types: Plasma
-
TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23.
Authors: Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M
Eur. J. Immunol., 2009-05-01;39(5):1221-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells.
Authors: Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G
Circulation, 2009-03-02;119(10):1424-32.
Species: Human
Sample Types: Plasma
-
Detuning CD8+ T lymphocytes by down-regulation of the activating receptor NKG2D: role of NKG2D ligands released by activated T cells.
Authors: Cerboni C, Ardolino M, Santoni A, Zingoni A
Blood, 2009-01-05;113(13):2955-64.
Species: Human
Sample Types: Cell Culture Supernates
-
Der p 1 suppresses indoleamine 2, 3-dioxygenase in dendritic cells from house dust mite-sensitive patients with asthma.
Authors: Maneechotesuwan K, Wamanuttajinda V, Kasetsinsombat K, Huabprasert S, Yaikwawong M, Barnes PJ, Wongkajornsilp A
J. Allergy Clin. Immunol., 2008-12-05;123(1):239-48.
Species: Human
Sample Types: Cell Culture Supernates
-
Helminth infections associated with multiple sclerosis induce regulatory B cells.
Authors: Correale J, Farez M, Razzitte G
Ann. Neurol., 2008-08-01;64(2):187-99.
Species: Human
Sample Types: Cell Culture Supernates
-
Adenosine receptors in regulation of dendritic cell differentiation and function.
Authors: Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM
Blood, 2008-06-17;112(5):1822-31.
Species: Human
Sample Types: Cell Culture Supernates
-
alpha1beta1 integrin and interleukin-7 receptor up-regulate the expression of RANKL in human T cells and enhance their osteoclastogenic function.
Authors: Gendron S, Boisvert M, Chetoui N, Aoudjit F
Immunology, 2008-05-13;125(0):359.
Species: Human
Sample Types: Cell Culture Supernates
-
Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12.
Authors: Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, Moyle M
J. Immunol., 2008-05-01;180(9):6000-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Diminished frequency and function of CD4+CD25high regulatory T cells associated with active uveitis in Vogt-Koyanagi-Harada syndrome.
Authors: Chen L, Yang P, Zhou H, He H, Ren X, Chi W, Wang L, Kijlstra A
Invest. Ophthalmol. Vis. Sci., 2008-04-17;49(8):3475-82.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-24 modulates IFN-gamma expression in patients with tuberculosis.
Authors: Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, Clayberger C
Immunol. Lett., 2007-12-26;117(1):57-62.
Species: Human
Sample Types: Cell Culture Supernates
-
The differential effect of Eriobotrya japonica hydrophilic leaf extract on cytokines production and modulation.
Authors: Matalka KZ, Ali D, Khawad AE, Qa'dan F
Cytokine, 2007-11-26;40(3):235-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Neuroimmune crosstalk in asthma: dual role of the neurotrophin receptor p75NTR.
Authors: Nassenstein C, Kammertoens T, Veres TZ, Uckert W, Spies E, Fuchs B, Krug N, Braun A
J. Allergy Clin. Immunol., 2007-08-22;120(5):1089-96.
Species: Mouse
Sample Types: BALF
-
Chimeric NKG2D receptor-bearing T cells as immunotherapy for ovarian cancer.
Authors: Barber A, Zhang T, DeMars LR, Conejo-Garcia J, Roby KF, Sentman CL
Cancer Res., 2007-05-15;67(10):5003-8.
Species: Human
Sample Types: Cell Culture Supernates
-
The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity.
Authors: Lauzon NM, Mian F, MacKenzie R, Ashkar AA
Cell. Immunol., 2006-10-17;241(2):102-12.
Species: Human
Sample Types: Cell Culture Supernates
-
A combination of E. coli DNA fragments and modified lipopolysaccharides as a cancer immunotherapy.
Authors: Cho YJ, Ahn BY, Lee NG, Lee DH, Kim DS
Vaccine, 2006-05-06;24(31):5862-71.
Species: Human
Sample Types: Cell Culture Supernates
-
An investigation of auto-reactivity after head injury.
Authors: Cox AL, Coles AJ, Nortje J, Bradley PG, Chatfield DA, Thompson SJ, Menon DK
J. Neuroimmunol., 2006-03-06;174(1):180-6.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate.
Authors: Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E
J. Immunol., 2004-07-15;173(2):1463-76.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of type 2 cytokines with hepatic fibrosis in human Schistosoma mansoni infection.
Authors: de Jesus AR, Magalhaes A, Miranda DG, Miranda RG, Araujo MI, de Jesus AA, Silva A, Santana LB, Pearce E, Carvalho EM
Infect. Immun., 2004-06-01;72(6):3391-7.
Species: Human
Sample Types: Cell Culture Supernates
-
The Toll-like receptor-2/6 agonist macrophage-activating lipopeptide-2 cooperates with IFN-gamma to reverse the Th2 skew in an in vitro allergy model.
Authors: Weigt H, Muhlradt PF, Larbig M, Krug N, Braun A
J. Immunol., 2004-05-15;172(10):6080-6.
Species: Human
Sample Types: Cell Culture Supernates
-
Immune responses in normal Indian langur monkeys (Presbytis entellus)--a primate model for visceral leishmaniasis.
Authors: Misra A, Dube A, Naik S
J. Med. Primatol., 2004-04-01;33(2):65-9.
Species: Primate - Presbytis entellus (Indian langur)
Sample Types: Cell Culture Supernates
-
Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain.
Authors: Finney HM, Akbar AN, Lawson AD
J. Immunol., 2004-01-01;172(1):104-13.
Species: Human
Sample Types: Cell Culture Supernates
-
Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis.
Authors: Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T
Proc. Natl. Acad. Sci. U.S.A., 2003-07-23;100(16):9452-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Toxoplasma gondii induces granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor secretion by human fibroblasts: implications for neutrophil apoptosis.
Authors: Channon JY, Miselis KA, Minns LA, Dutta C, Kasper LH
Infect. Immun., 2002-11-01;70(11):6048-57.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human IFN-gamma DuoSet ELISA
Average Rating: 4.4 (Based on 15 Reviews)
Have you used Human IFN-gamma DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
EDTA plasma, used neat
Plasma tested at 2-fold dilution but appears to cause a matrix effect.
Detection of IFN-g produced by BCMA-specific CAR-T cells in response to BCMA-negative and BCMA-positive hematopoietic cell lines.
Nice linear curve. Good detection rate for the tissue culture supernatant